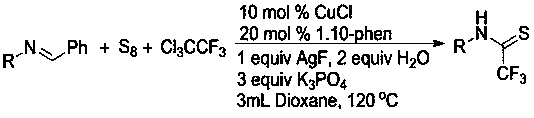Method for preparing thiotrifluoroacetamide compound
A technology for thiotrifluoroacetamide and compounds, which is applied in the field of preparing thiotrifluoroacetamide compounds, can solve the problem of difficult synthesis of trifluoromethyl-containing thioamide compounds and difficult preparation of trifluoromethyl group-containing compounds Solve problems such as thioamide compounds, and achieve the effects of simple operation, easy-to-obtain products, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
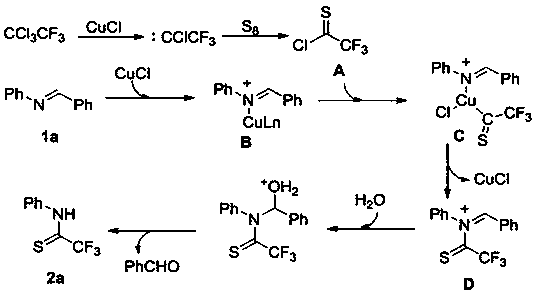
[0020] A method for preparing sulfur-containing trifluoroacetamide compounds, comprising the following steps: using (E)-N,1-diphenylimine as a substrate, adding elemental sulfur to the substrate, 1,1,1 -Trifluorotrichloroethane, the molar ratio of the three is 1:1:5. By adding 10% cuprous chloride, 20% 1.10-phenanthroline, 100% additive silver fluoride, 300% anhydrous potassium phosphate, 200% distilled water, in the reaction solvent of 1.4-dioxane , and reacted for 4 hours at a temperature of 120°C; the chemical reaction formula is as follows:
[0021]
[0022] The R 1 For phenyl, 4-methylphenyl, 2-methylphenyl, 4-tert-butyl, 4-biphenyl, 4-phenoxyphenyl, 4-methoxyphenyl, 4-methylthio phenyl, 4-fluorophenyl, 4-chlorophenyl, 3-chlorophenyl, 4-bromophenyl, 4-iodophenyl, 4-trifluoromethylphenyl, 4-methylformylphenyl , one of 2-naphthyl, phenethyl, and cyclopentyl;
[0023] After the reaction is finished, after cooling, the reaction solution is filtered to obtain the filtra...
specific Embodiment 1
[0024] Specific Example 1: 36.2 mg (0.2 mmol) (E)-N,1-diphenylimine, 51.2 mg (0.2 mmol) elemental sulfur, 187.0 mg (1.0 mmol) 1,1,1-trifluorotri Ethyl chloride, 2.0 mg (0.02 mmol) cuprous chloride, 7.2 mg (0.04 mmol) 1.10-phenanthroline, 25.2 mg (0.2 mmol) silver fluoride, 127.2 mg (0.6 mmol) potassium phosphate, 7.2 mg (0.4 mmol) distilled water was added to 3 mL of 1.4-dioxane solvent. Reacted at 120°C for 4 hours, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove the solvent, and the residue was subjected to silica gel column chromatography, washed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 15:1, according to The effluent was collected by actual gradient, detected by TLC, the effluent containing the product was combined, the solvent was distilled off by a rotary evaporator, and dried in vacuo to obtain 28.3 mg of yellow oily 2,2,2-trifluoro-N-phenylthioacetamide, the yield 69%. Yellow oil. 1 H NMR...
specific Embodiment 2
[0025] Specific example two: 39.0 mg (0.2 mmol) (E)-1-phenyl-N-(4-tolyl) methyl imine, 51.2 mg (0.2 mmol) elemental sulfur, 187.0 mg (1.0 mmol) 1, 1,1-Trifluorotrichloroethane, 2.0 mg (0.02 mmol) cuprous chloride, 7.2 mg (0.04 mmol) 1.10-phenanthroline, 25.2 mg (0.2 mmol) silver fluoride, 127.2 mg (0.6 mmol) Potassium phosphate, 7.2 mg (0.4 mmol) of distilled water, was added to 3 mL of 1.4-dioxane solvent. Reacted at 120°C for 4 hours, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove the solvent, and the residue was subjected to silica gel column chromatography, washed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 15:1, according to The effluent was collected in the actual gradient, detected by TLC, the effluent containing the product was combined, the solvent was distilled off by a rotary evaporator, and the yellow solid was obtained by vacuum drying, 26.3 mg of 2,2,2-trifluoro-N-(4-tolyl)thio Acetamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com