Portunus trituberculatus mannose-binding lectin PtMBL gene, encoding protein thereof and application
A technology of Portunus trituberculatus and its encoded protein, which is applied in the field of Portunus trituberculatus mannose-binding lectin PtMBL gene and its encoded protein, and can solve the problems that Portunus trituberculatus has little research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
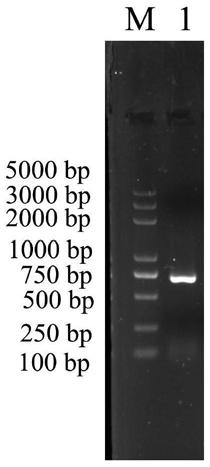
[0037] The base sequence of the mannose-binding lectin PtMBL gene of Portunus trituberculatus is shown in SEQ ID No.1.
[0038] The sequence listing SEQ ID No.1 is:
[0039]GTTCCTCAGGTTTGATGCTCGGGGACTTCTCCTCCGCAAGATGAAGGTCGCTGTCGTCCTGCTGTCGTGCCTCGCCTTCGCCGCCTCCTCCCGGCGTCCCTACCCGAGCCGCTACCCAAGCTCCGGCGGCGGCTTCCCTGGCAGAGGCTCAGTTATCGTCTTCCCCGACGAGGTCAAGGGCGGTGGAGGAAGCGGGGGCCACGTCCACCCTGGAGTTGGTGTTGGGAATATTTACCCCCCACCAGTCGGTCATGGTGGCGAGCCTCTGGTCACTAGCCACTGCCCACGCGCTATTTCCAAAGATGTCCACGGCACCTTCCTGGGACACAACTACCACTTCTCTTGGTGCGCTGATGGCGGCCAGCGCTACACTTGGGAGGCCGCCCGCGACTACTGCACCAGGCTGGGCCCCGGCTGGTACCCTGTGGCCATCGAAAGTAGGGATGAAGACAACTACATCATCGACATTGTTGGCAGCCACCAATCTCCCTGGATCTGGACTGGCGGGAACACTTTGAGCAACACTAACTACGTCTGGCAATGGCTGGATGGAAGTTCTCTCATCTACACTAACTGGGGACAGACTGGATCATTGGACAGACCACAGCCAGACAACGCCGAAAACAACAACGAACGGTGCCTGGCTATCCTCAACCAGTTCTACGCAGGAGACTTCATTACTTTCCACGACATCGGATGCCATCACACCAAACCAACCATCTGCGAGAACTCTAATGTCCAGGTTCCCGTCCCCGTGCCCCAGTATGGTTAAGAGCGATGGCTAAGGTATAATAGGTTCGGAGATAGTGACTGTCCAACG...
Embodiment 2
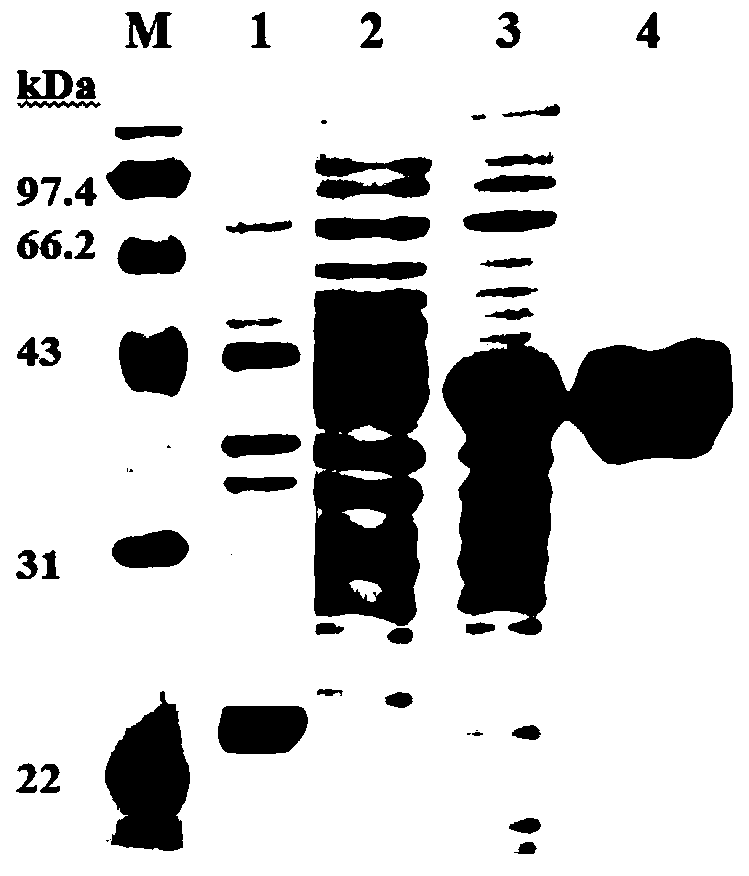
[0071] The base sequence of Portunus trituberculatus mannose-binding lectin PtMBL is described in SEQ ID No. 1 in the sequence listing, and the amino acid sequence is described in SEQ ID No. 2 in the sequence listing.
[0072] The sequence listing SEQ ID No.2 is:
[0073]MKVAVVLLSCLAFAASSRRPYPSRYPSSGGGFPGRGSVIVFPDEVKGGGGSGGHVHPGVGVGNIYPPPVGHGGEPLVTSHCPRAISKDVHGTFLGHNYHFSWCADGGQRYTWEAARDYCTRLGPGWYPVAIESRDEDNYIIDIVGSHQSPWIWTGGNTLSNTNYVWQWLDGSSLIYTNWGQTGSLDRPQPDNAENNNERCLAILNQFYAGDFITFHDIGCHHTKPTICENSNVQVPVPVPQYG
[0074] It has a complete coded protein containing 243 amino acids, 1-16 amino acids of the coding sequence are signal peptides, the mature peptide contains 227 amino acids, the predicted molecular weight is 24.87kDa, and the isoelectric point is 5.95. The mature peptide lacks the cysteine-rich, collagen-like, and neck regions reported in vertebrates, but has a typical CRD domain (92-229) containing four cysteine residues capable of forming Two disulfide bonds (C 11...
Embodiment 3
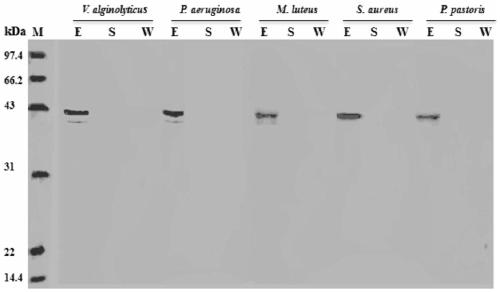
[0078] 1. Antibacterial test in vitro of recombinant protein of Portunus trituberculatus mannose-binding lectin PtMBL:
[0079] Culture and preparation of microorganisms: culture Vibrio alginolyticus in TSB medium at 28°C, Pseudomonas aeruginosa in TSB medium at 37°C, Staphylococcus aureus in LB medium at 37°C, Micrococcus luteus in LB medium Cultivate at 37°C, Pichia pastoris was cultured with YPD medium at 28°C, and each of the above strains was cultured on a shaker at 220rpm / min to make the bacterial concentration reach the logarithmic growth phase, and the bacteria were diluted with 50mM Tris-HCl (pH=8.0) buffer respectively. body, so that the number of colonies per milliliter of bacterial liquid is about 1×10 3 indivual.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com