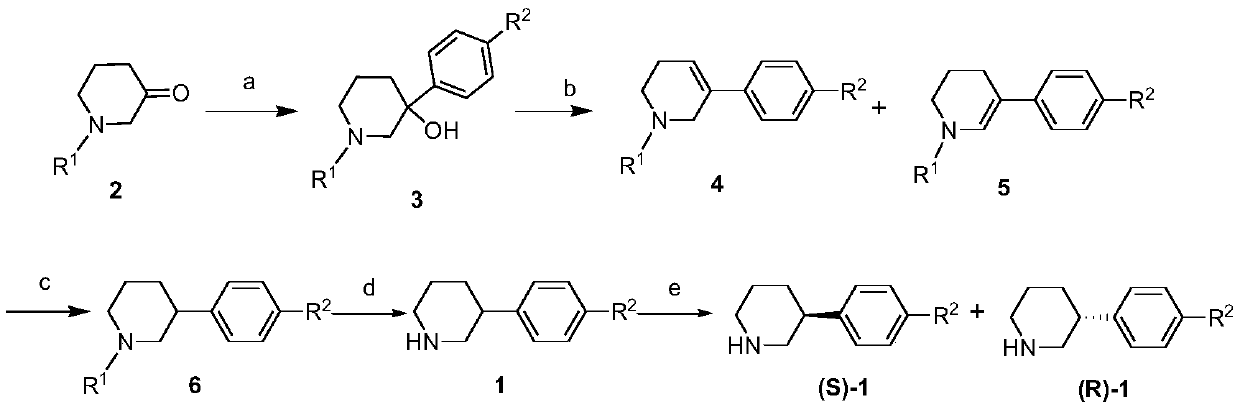
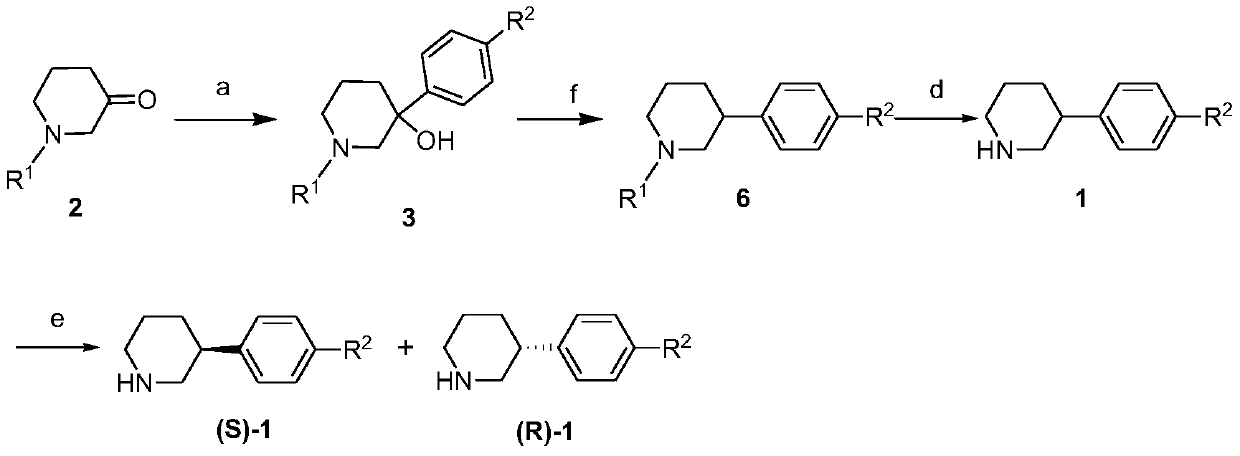
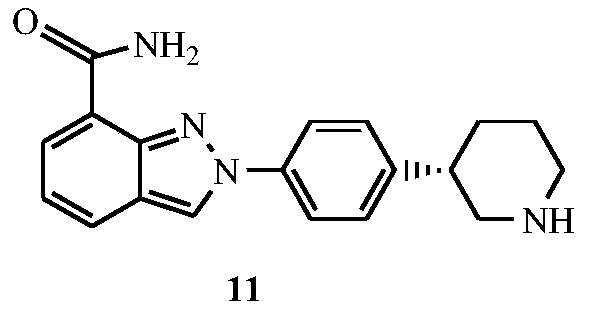
Synthesis method of (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method of chiral intermediates of niraparib
A chiral intermediate, phenylpiperidine technology, applied in the field of synthesis of chiral intermediates, can solve the problem of high cost of synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3-1)
[0088]
[0089] Phenylmagnesium bromide Grignard reagent (2mol / L in THF, 240mL) and anhydrous tetrahydrofuran (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3- Piperidone (2-1, 60.0g, 317.0mmol) was diluted with anhydrous tetrahydrofuran (300mL), added to the dropping funnel, and slowly added dropwise to the reaction flask, the temperature was controlled at 0-5°C, and the dripping was completed in 60 minutes. After the dropwise addition was completed, the stirring reaction was continued for 1 hour. TLC detected that the raw materials had reacted completely. Under stirring, a saturated ammonium chloride aqueous solution (300mL) was added, and then extracted with ethyl acetate (200mL×3). After three extractions, the organic layers were combined and added to An appropriate amount of anhydrous sodium sulfate was dried, filtered, and...
Embodiment 2
[0090] Embodiment 2: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3-1)
[0091]
[0092] Phenylmagnesium bromide Grignard reagent (2mol / L in THF, 240mL) and anhydrous ether (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3- Piperidone (2-1, 60.0g, 317.0mmol) was diluted with anhydrous diethyl ether (300mL), added to the dropping funnel, slowly added dropwise to the reaction flask, and the temperature was controlled at 0-5°C, and the dripping was completed in 60 minutes. After the dropwise addition was completed, the stirring reaction was continued for 1 hour. TLC detected that the raw materials had reacted completely. Under stirring, a saturated ammonium chloride aqueous solution (300mL) was added, and then extracted with ethyl acetate (200mL×3). After three extractions, the organic layers were combined, and then An appropriate amount of anhydrous sodium sulfate was added to the combined organic layer...
Embodiment 3
[0093] Embodiment 3: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3-1)
[0094]
[0095] Phenylmagnesium chloride Grignard reagent (2mol / L in THF, 240mL) and anhydrous tetrahydrofuran (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3-piperidine Ketone (2,60.0g, 317.0mmol) was diluted with anhydrous tetrahydrofuran (300mL), added to the dropping funnel, and slowly added dropwise to the reaction flask, the temperature was controlled at 0-5°C, and the drop was completed after 50 minutes. Continue to stir and react for 1.5 hours, TLC detects that the raw materials have reacted completely, add saturated ammonium chloride aqueous solution (300mL) under stirring, and then extract with ethyl acetate (200mL×3), after extracting three times, combine the organic layers, and then add to the combined organic An appropriate amount of anhydrous sodium sulfate was added to the layer to dry, filtered, and the solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com