Preparation method of rosuvastatin calcium intermediate
A compound, the technology of isopropyl, applied in the field of drug synthesis, can solve the problems that the synthesis process is not suitable for industrialization, there is no commercial supplier, and the yield of the synthesis process product is low, so as to avoid unstable brominated intermediates , suitable for large-scale production, high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
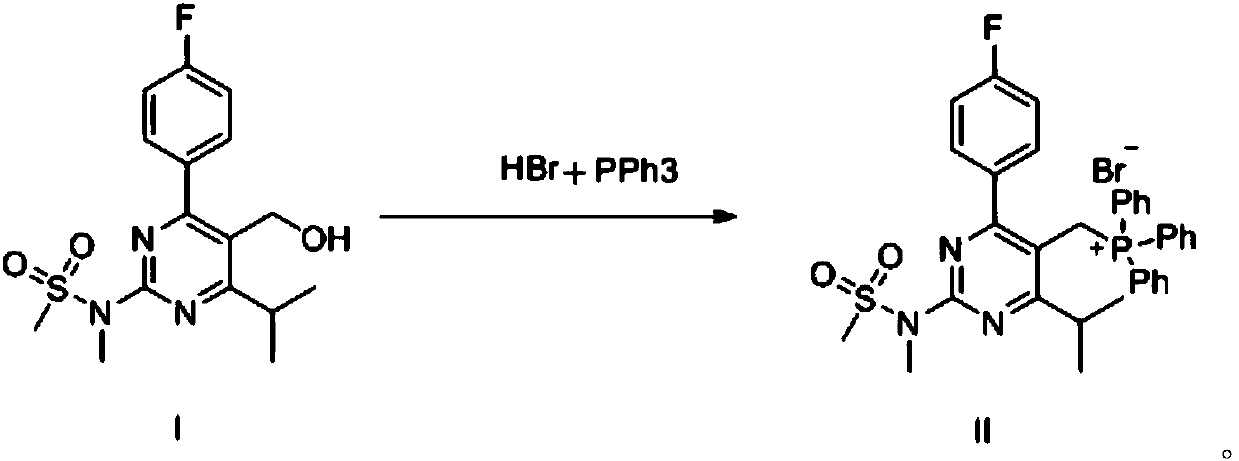
[0031] 3.5g of 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidine-5-methanol (compound I), 2.6g tris Phenylphosphine and 30ml of toluene were added to a 100ml three-necked flask, and 1.84g of 48% hydrobromic acid was added, heated to reflux and stirred. After the reaction was completed, it was cooled and crystallized, and dried to obtain a white solid, which was [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonamido)-5- Pyrimidinyl]triphenylphosphine bromide (compound II). Yield: 85%; Purity: 99.0%.
[0032] 1 H NMR (400MHz, DMSO-d6): δ7.87(m, 3H), 7.63(m, 6H), 7.27(m, 8H), 7.13(t, J=8.4Hz, 2H), 5.08(d, J =13.6Hz,2H),3.49(s,3H),3.40(s,3H),2.86(m,1H),0.79(d,J=4.4Hz,6H)
Embodiment 2
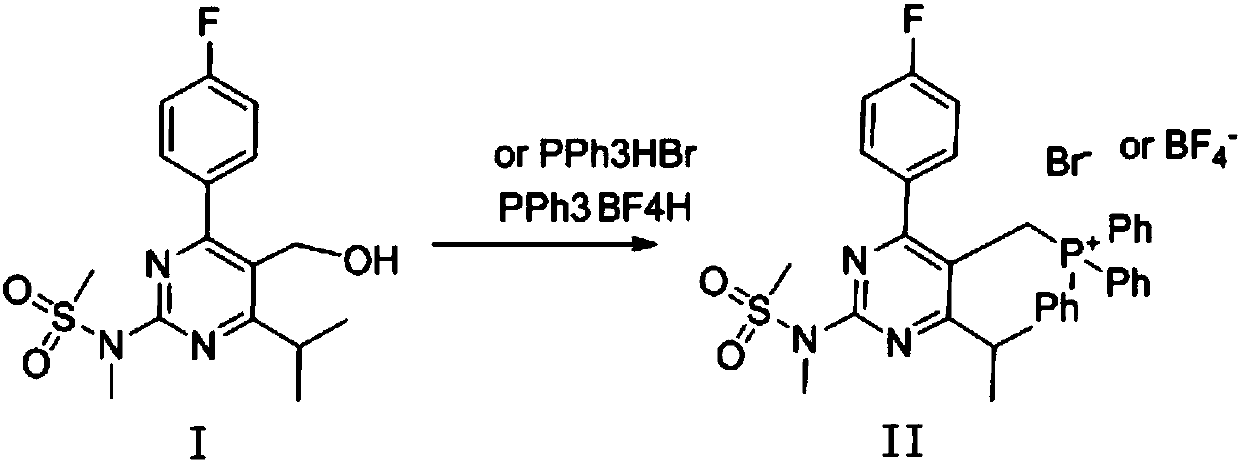
[0034] 3.5g of 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidine-5-methanol (compound I), 2.6g tris Phenylphosphine and 30ml of acetonitrile were added to a 100ml three-necked flask, and 1.84g of 48% hydrobromic acid was added, heated to reflux and stirred. After the reaction was completed, it was concentrated to dryness to obtain a white solid, which was [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonamido)-5-pyrimidinyl ] Triphenylphosphine bromide (compound II). Yield: 98%; Purity: 96.0%.
Embodiment 3-10
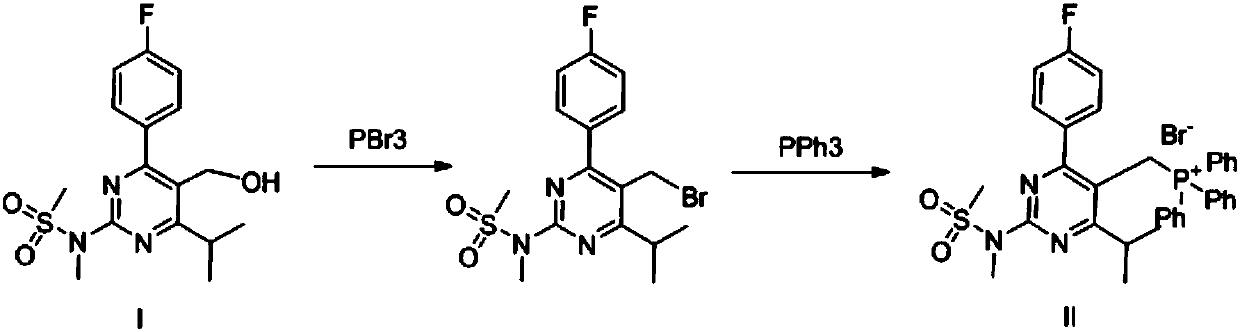
[0036] According to the method shown in Example 1, Compound II was prepared by adjusting the ratio between Compound I, hydrogen bromide and triphenylphosphine in hydrobromic acid and the type of solvent. The results are shown in Table 1.
[0037] Preparation of Table 1 Compound II
[0038]
[0039] As can be seen from Table 1, considering the comprehensive factors of the yield and purity of compound II, the experimental results of acetonitrile or acetonitrile / water mixed solvent as solvent are better than dichloromethane and tetrahydrofuran.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com