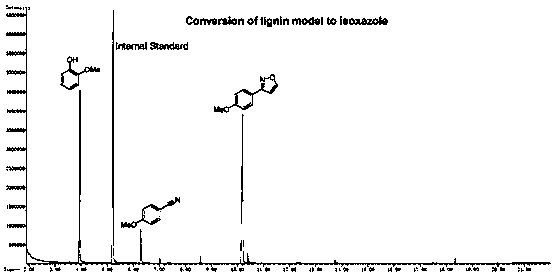
Method for converting lignin into isoxazole and aromatic nitrile
A technology of lignin and isoxazole, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of simple conversion method, expanded application prospects, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 15mL pressure bottle, add 1mL methanol, 0.1mmol 3-hydroxy-2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)propyl-1-one and 0.3mmol hydrochloric acid Hydroxylamine was filled with nitrogen to replace the atmosphere, airtight, stirred and reacted at 120°C for 12 hours, after the reaction, centrifuged, and the product was detected by chromatography. The conversion rate and yield are shown in Table 1.
Embodiment 2
[0038] In a 15mL pressure bottle, add 1mL methanol, 0.1mmol 3-hydroxy-2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)propyl-1-one, 0.3mmol hydrochloric acid Hydroxylamine and 0.2 mmol MgO were filled with nitrogen to replace the atmosphere, airtight, and stirred at 120°C for 12 hours. After the reaction, centrifuged, and the product was detected by chromatography. The conversion rate and yield are shown in Table 1.
Embodiment 3
[0040] In a 15mL pressure bottle, add 1mL methanol, 0.1mmol 3-hydroxy-2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)propyl-1-one, 0.3mmol hydrochloric acid Hydroxylamine and 0.2 mmol ZnO were filled with nitrogen to replace the atmosphere, airtight, and stirred at 120°C for 12 hours. After the reaction, centrifuged and chromatographically detected the product. The conversion rate and yield are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com