Separation method and application of naphthol derivatives
A technology of naphthol derivatives and separation methods, which is applied in the field of separation of naphthol derivatives, can solve problems such as the inability to separate 1-isopropylamino-3-(2-naphthyloxy)-2-propanol, and achieve Good separation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Instruments: Agilent 1260 high performance liquid chromatography, electronic analytical balance, pH meter
[0060] The ratio of the mobile phase is a mixture of acetonitrile-water-sulfuric acid (50:50:0.1) (take 1.6g of sodium lauryl sulfate and 0.31g of tetrabutylammonium dihydrogen phosphate and dissolve it in 1000ml of the mixture, use 2mol / L sodium hydroxide solution adjusts the pH value to 3.3);
[0061] The chromatographic column is Agilent SB C18 (250mm×4.6mm×5μm);
[0062] The flow rate is 1.2ml / min;
[0063] The detection wavelength is 230nm;
[0064] Column temperature 40°C;
[0065] Accurately weigh 3-(1-naphthyloxy)-1,2-propanediol, 1-isopropylamino-3-(2-naphthyloxy)-2-propanol, 1-naphthylglycidyl ether , 1,2-di-isopropylamino-3-(1-naphthyloxy)-2-propanol, 1,3-di-(1-naphthyloxy)propan-2-ol and 3,3'- An appropriate amount of (isopropylamino)-di-(1-naphthyloxy)propan-2-ol, the mobile phase is diluted to contain 3-(1-naphthyloxy)-1,2-propanediol, 1- Isop...
Embodiment 2
[0108] All the other operations are as in Example 1, only the mobile phase is changed to the mixed solution of acetonitrile-water-sulfuric acid (51:49:0.1) (sodium lauryl sulfate 1.6g and tetrabutylammonium dihydrogen phosphate 0.31g are dissolved in In the 1000ml mixed solution, adjust the pH value to 3.3 with 2mol / L sodium hydroxide solution), and the sample adopts the resolution solution and the test solution.
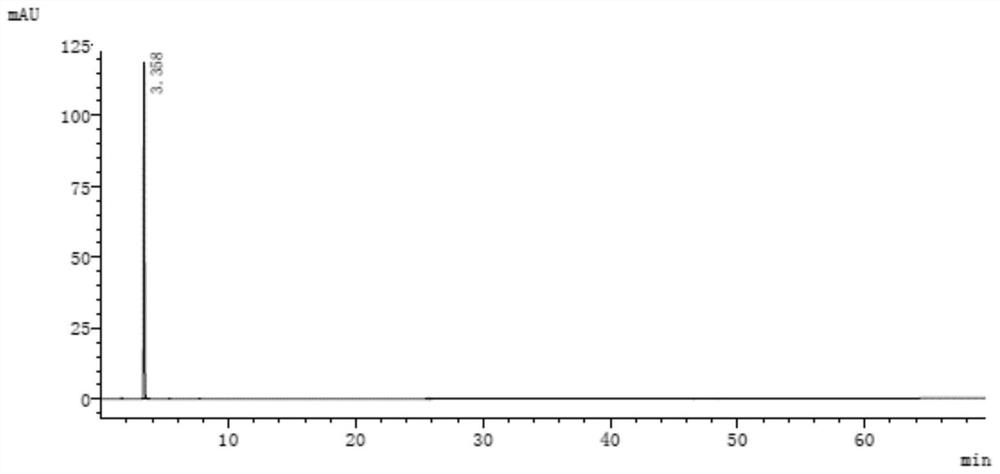
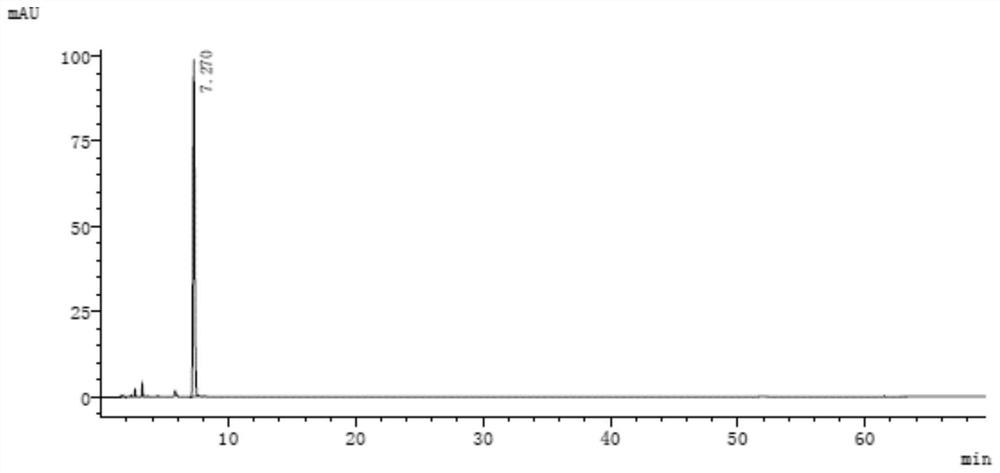
[0109] See the attached chromatogram for details Figure 9 and 10 .
[0110] The analytical detection result of resolution solution is as shown in table 9:
[0111] Table 9
[0112]
[0113] Among them, the peak numbers are assigned as follows:
[0114] Peak number 1 is 3-(1-naphthyloxy)-1,2-propanediol;
[0115] Peak number 2 is 1-isopropylamino-3-(2-naphthyloxy)-2-propanol;
[0116] Peak No. 3 is propranolol hydrochloride;
[0117] Peak number 4 is 1-naphthyl glycidyl ether;
[0118] Peak number 5 is 1,2-di-isopropylamino-3-(1-naphthyloxy)-2-propanol; ...
Embodiment 3
[0130] All the other operations are as in Example 1, only the mobile phase is changed to the mixed solution of acetonitrile-water-sulfuric acid (49:51:0.1) (sodium lauryl sulfate 1.6g and tetrabutylammonium dihydrogen phosphate 0.31g are dissolved in In the 1000ml mixed solution, adjust the pH value to 3.3 with 2mol / L sodium hydroxide solution), and the sample adopts the resolution solution and the test solution.
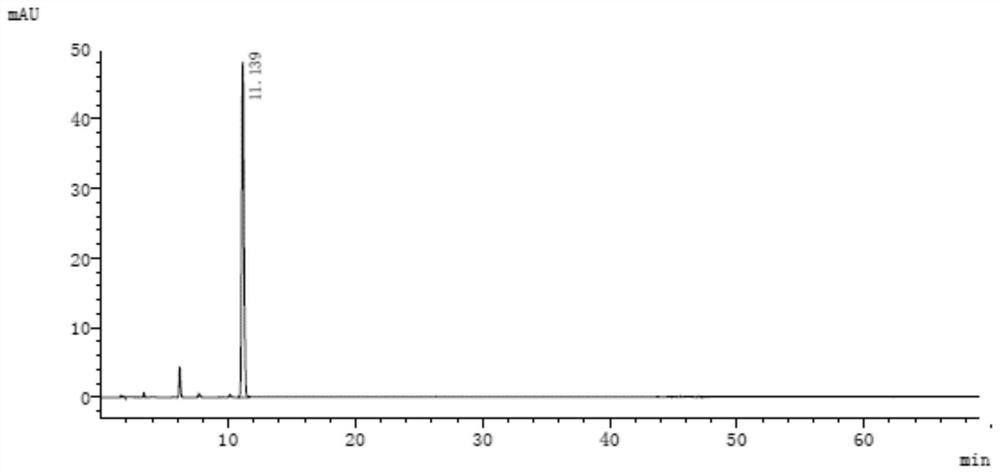
[0131] See the attached chromatogram for details Figure 11 and 12 .
[0132] The analytical detection result of resolution solution is as shown in table 11:
[0133] Table 11
[0134]
[0135]
[0136] Among them, the peak numbers are assigned as follows:
[0137] Peak number 1 is 3-(1-naphthyloxy)-1,2-propanediol;
[0138] Peak number 2 is 1-isopropylamino-3-(2-naphthyloxy)-2-propanol;
[0139] Peak No. 3 is propranolol hydrochloride;
[0140] Peak number 4 is 1-naphthyl glycidyl ether;
[0141] Peak number 5 is 1,2-di-isopropylamino-3-(1-naphthyloxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com