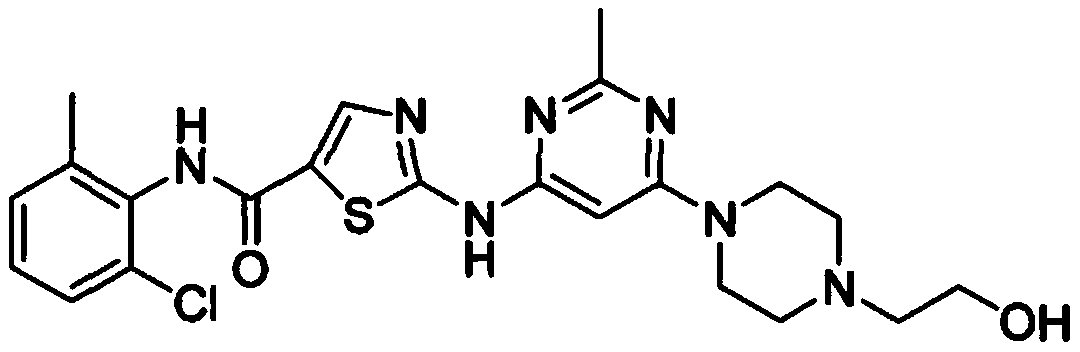
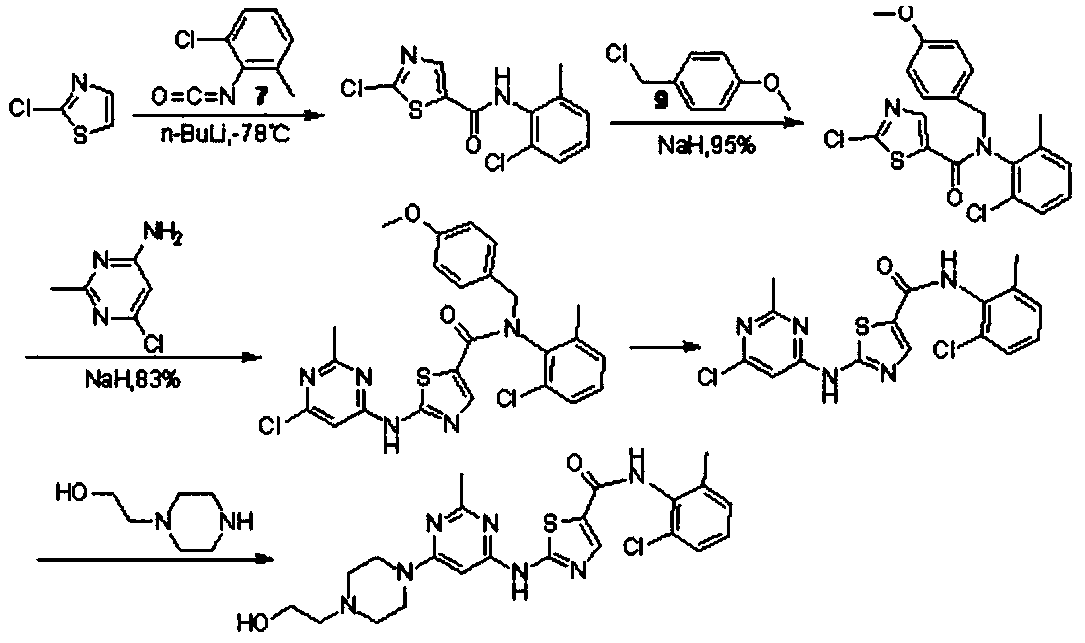
Dasatinib preparation method
A technology of dasatinib and quantitative ratio, which is applied in the field of drug synthesis, can solve the problems of long synthetic routes, impure intermediate products, and large environmental pollution, and achieve the effects of increased yield, short reaction time, and no environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis of compound 3
[0044] Dissolve 30mmol of ethyl 3-oxopropionate and 37.5mmol of sodium methoxide in 80mL of tetrahydrofuran. After stirring at room temperature for 10 minutes, add 27mmol of 2-chloro-6-methylaniline, heat and reflux for 1 hour. After the reaction is over, cool to room temperature. Add 60mL of tetrahydrofuran with 81mmol of copper bromide dissolved in it, heat to reflux for 2h, filter while hot, wash the filter cake with 50mL of hot tetrahydrofuran, combine the filtrate with the washing liquid, wash with water until neutral, distill under reduced pressure to remove the solvent, and pour the residue into Stir in 50 mL of ice water for 0.5 h, filter under reduced pressure, wash the filter cake with ice water, and dry to obtain 7.19 g of compound 3 with a yield of 91.60% and a purity of 99.92%.
Embodiment 2
[0045] Example 2: Synthesis of Compound 3
[0046] Dissolve 30mmol of ethyl 3-oxopropionate and 39mmol of sodium methoxide in 80mL of tetrahydrofuran. After stirring for 10min at room temperature, add 30mmol of 2-chloro-6-methylaniline and heat to reflux for 1h. After the reaction, cool to room temperature and add 60mL of tetrahydrofuran with 84mmol of copper bromide dissolved in it, heated to reflux for 2h, filtered while hot, the filter cake was washed with 50mL of hot tetrahydrofuran, the filtrate was combined with the washing liquid, washed with water until neutral, the solvent was distilled off under reduced pressure, and the residue was poured into 50mL The mixture was stirred in ice water for 0.5 h, filtered under reduced pressure, and the filter cake was washed with ice water and dried to obtain 7.89 g of compound 3 with a yield of 90.43% and a purity of 99.90%.
Embodiment 3
[0047] Example 3: Synthesis of Compound 3
[0048] Dissolve 30mmol of ethyl 3-oxopropionate and 36mmol of sodium methoxide in 80mL of tetrahydrofuran. After stirring at room temperature for 10 minutes, add 24mmol of 2-chloro-6-methylaniline. The temperature is heated to reflux for 1 hour. After the reaction is over, it is cooled to room temperature and added 60mL of tetrahydrofuran with 78mmol of copper bromide dissolved in it, heated to reflux for 2h, filtered while hot, the filter cake was washed with 50mL of hot tetrahydrofuran, the filtrate was combined with the washing liquid, washed with water until neutral, the solvent was distilled off under reduced pressure, and the residue was poured into 50mL Stir in ice water for 0.5 h, filter under reduced pressure, wash the filter cake with ice water, and dry to obtain 5.99 g of compound 3 with a yield of 85.72% and a purity of 99.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com