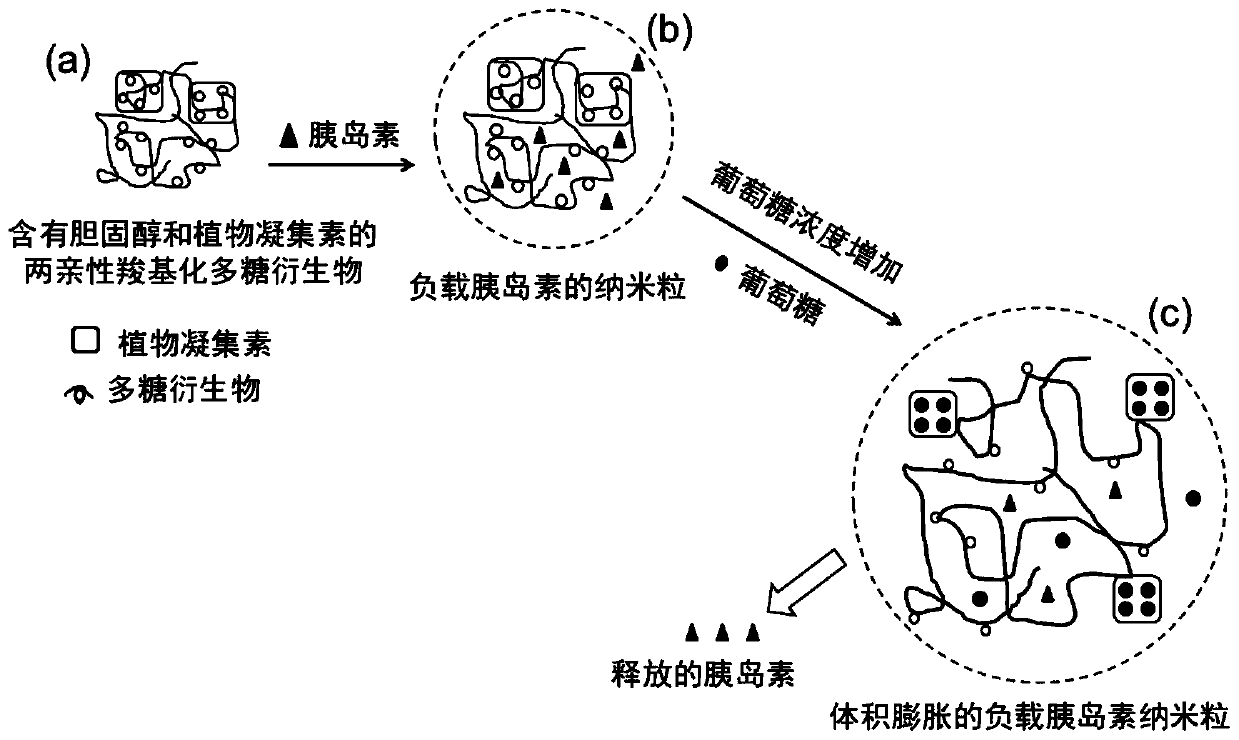
Amphiphilic polysaccharide derivative containing cholesterol and phytolectin group as well as preparation method and application of amphiphilic polysaccharide derivative
A technology of amphiphilic polysaccharides and plant lectins, applied in the field of amphiphilic polysaccharide derivatives and their preparation, can solve the problems of difficult subcutaneous or intravenous injection, large size, etc., and achieve the effect of intelligent release of therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1. Synthesis of amphipathic amylose derivatives containing cholesterol and lectin groups
[0076] (1) Weigh 0.1 g of carboxylated amylose derivatives, dissolve in 20 ml of dimethyl sulfoxide, stir and dissolve in an oil bath at 15° C. for 2 hours;
[0077] (2) Weigh 0.2 g of cholesterol in 10 ml of ethanol, slowly add it dropwise to the solution of step (1), then weigh 0.5 g of DCC and 1.1 g of DMAP, dissolve it in 100 ml of organic solvent, and slowly add it dropwise to step (1) ) solution, continue to stir and react in an oil bath at 15°C for 1 hour;
[0078] (3) After the above reaction was completed, 1 mL of acetone was added dropwise to the reaction solution to precipitate amphipathic amylose derivatives containing cholesterol, and the precipitate was collected by centrifugation (5000 rpm, 5 min). Dissolve the precipitate in 20 mL of ethanol, add 1-20 mL of acetone dropwise to precipitate amphipathic amylose derivatives containing cholesterol, centrifuge (5000 rpm...
Embodiment 2
[0099] 1. Synthesis of amphiphilic glycogen derivatives containing cholesterol and lectin groups
[0100] (1) Weigh 0.3 g of carboxylated glycogen derivatives, dissolve in 15 ml of acetonitrile, stir and dissolve in an oil bath at 40° C. for 5 hours;
[0101] (2) Weigh 0.2 g of cholesterol in 10 ml of acetone, slowly add it dropwise to the solution of step (1), then weigh 1.0 g of DCC and 1.0 g of DMAP, dissolve it in 100 ml of acetone, and slowly add dropwise to the solution of step (1) solution, the stirring reaction was continued for 5 hours.
[0102] (3) After the above reaction was completed, 1 mL of acetone was added dropwise to the reaction solution to precipitate amphiphilic glycogen derivatives containing cholesterol, and the precipitate was collected by centrifugation (5000 rpm, 5 min). Dissolve the precipitate in 20 mL of ethanol, add 1-20 mL of acetone dropwise to precipitate amphiphilic glycogen derivatives containing cholesterol, centrifuge (5000 rpm, 5 min), an...
Embodiment 3
[0115] 1. Synthesis of amphiphilic guar gum derivatives containing cholesterol and lectin groups
[0116] (1) Weigh 0.5 g of carboxylated guar gum derivatives, dissolve in 15 ml of formamide, stir and dissolve in an oil bath at 20° C. for 20 hours;
[0117] (2) Weigh 0.5 gram of cholesterol in 15 milliliters of dimethylformamide, slowly add it dropwise to the solution of step (1), then weigh 0.5 gram of DCC and 1.5 gram of DMAP and dissolve in 100 milliliters of dimethylformamide, Slowly added dropwise to the solution of step (1), and continued to stir and react for 20 hours.
[0118] (3) After the above reaction was completed, 1 mL of isopropanone was added dropwise to the reaction solution to precipitate amphiphilic glycogen derivatives containing cholesterol, and the precipitate was collected by centrifugation (5000 rpm, 5 min). Dissolve the precipitate in 20 mL of dimethylformamide, add 1-20 mL of isopropyl ketone dropwise to precipitate amphiphilic glycogen derivatives c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com