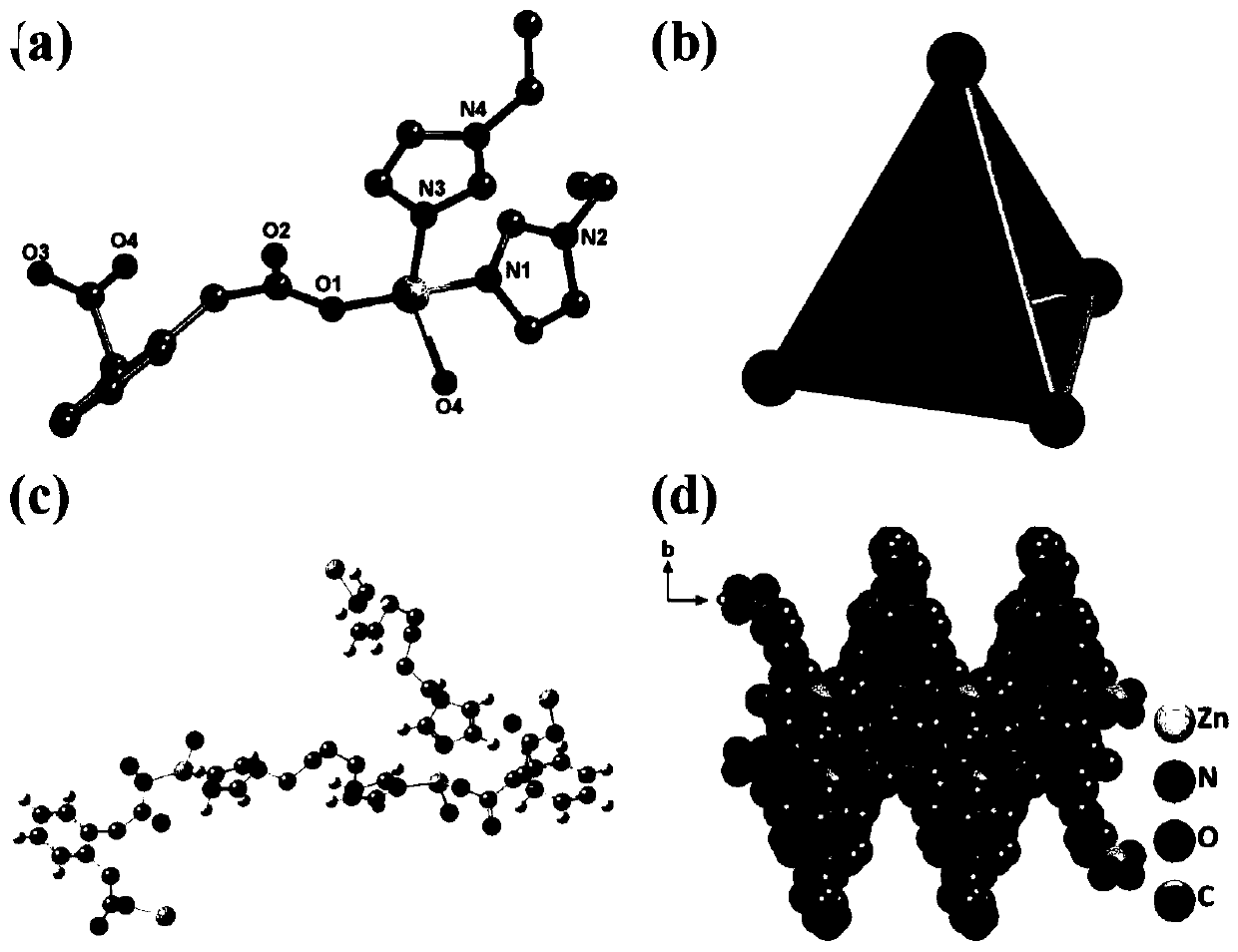
Application of MOF-Zn fluorescent sensor in detection of chloramphenicol as well as CHL detection method
A fluorescent sensor, chloramphenicol technology, applied in the field of detection of CHL, can solve the problems of high cost, poor repeatability and stability, long antibody development time, etc., and achieve the effect of low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares MOF-Zn solution:
[0034] First synthesize the ligand bbi: weigh a certain amount of imidazole (3.1-7.5g), NaOH (2.0-8.0g) and urea (3.6-4.5g) in H 2 O, DMA, DMSO, DMF, C 2 h 5 OH, CH 3 OH and other one or several combination solutions (10mL), fully stirred and mixed, reacted in an oil bath at 50-70°C for 1.5-12h, and then weighed a certain amount of 1,1-C 4 h 8 Cl 2 , 1,2-C 4 h 8 Cl 2 , 1,3-C 4 h 8 Cl 2, 1,4-C 4 h 8 Cl 2 , 2,2-C 4 h 8 Cl 2 and 2,3-C 4 h 8 Cl 2 (0.014~0.8mol) one or several combinations are added to the above solution several times, and after 5~8 hours, the solution is immersed in 500mLH 2 O, DMA, DMSO, DMF, C 2 h 5 OH,CH 3 OH and other mixtures of one or more combinations of ice and water, let it stand overnight to obtain a large amount of white needle-shaped product, which was filtered by suction to obtain white needle-shaped crystals, which were dried in a vacuum drying oven for later use.
[0035] Weigh ...
Embodiment 2
[0037] Example 2: MOF-Zn is used as a fluorescent probe for the detection of CHL
[0038] Add 100-150 μL of CHL solution to the MOF-Zn solution, and then add ERY, MTR, AMX, CFM, CED, AZM, CEC, PEN, AZL, FOX, CSU, ATM, CAZ, LIN, AMK, GEN, S XT, CRO. Image 6 The fluorescence of CHL on MOF-Zn solution decreased obviously.
[0039] In MOF-Zn solution, when the amount of CHL increased from 0 μL to 90 μL, the fluorescence intensity of MOF-Zn decreased monotonously and sharply, Figure 7 With the increase of CHL concentration, the fluorescence intensity of MOF-Zn solution was gradually quenched. This indicates that MOF-Zn can be highly sensitive to CHL as a fluorescent probe. By Stern-Volmer (SV) equation: I 0 / I=Ksv[Q]+1 can quantitatively explain the fluorescence quenching efficiency, and can also calculate the detection limit; where I 0 is the fluorescence intensity before the analyte, I is the fluorescence intensity after the analyte, and Ksv is the quenching constant (M -...
Embodiment 3
[0041] Example 3: MOF-Zn is used as a fluorescent probe for the practical detection of CHL
[0042] Get 1ml of commercially available chloramphenicol eye drops (Shandong Bausch & Lomb Freda Pharmaceutical Co., Ltd., containing 2.5 mg of chloramphenicol) and add it to a 100ml volumetric flask to dilute 100 times as the test solution. Take 50 μL of MOF-Zn solution, add 30 μL of the solution to be tested, and add 3 ml of distilled water; take another 50 μL of MOF-Zn solution, add 30 μL of distilled water, and then add 3 ml of distilled water. The two solutions were tested using a spectrofluorometer, from Figure 11 It can be seen that no fluorescence quenching occurred in the blank test, but significant fluorescence quenching occurred after adding a very small amount of chloramphenicol solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com