Method for preparation of bibenzyl compounds by photocatalytic one-step process
A compound and photocatalytic technology, applied in the preparation of organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of toxicity, large amount of catalyst usage, high cost of raw materials, etc., achieve simple and safe operation process, and broaden product categories , prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
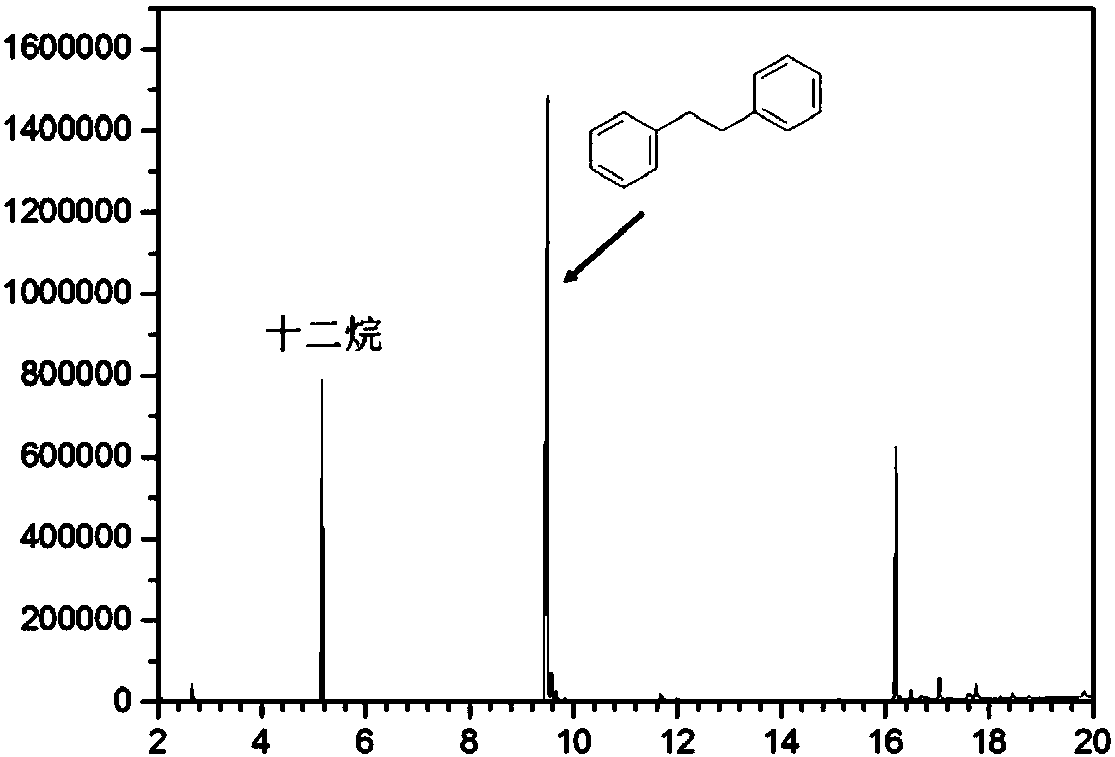
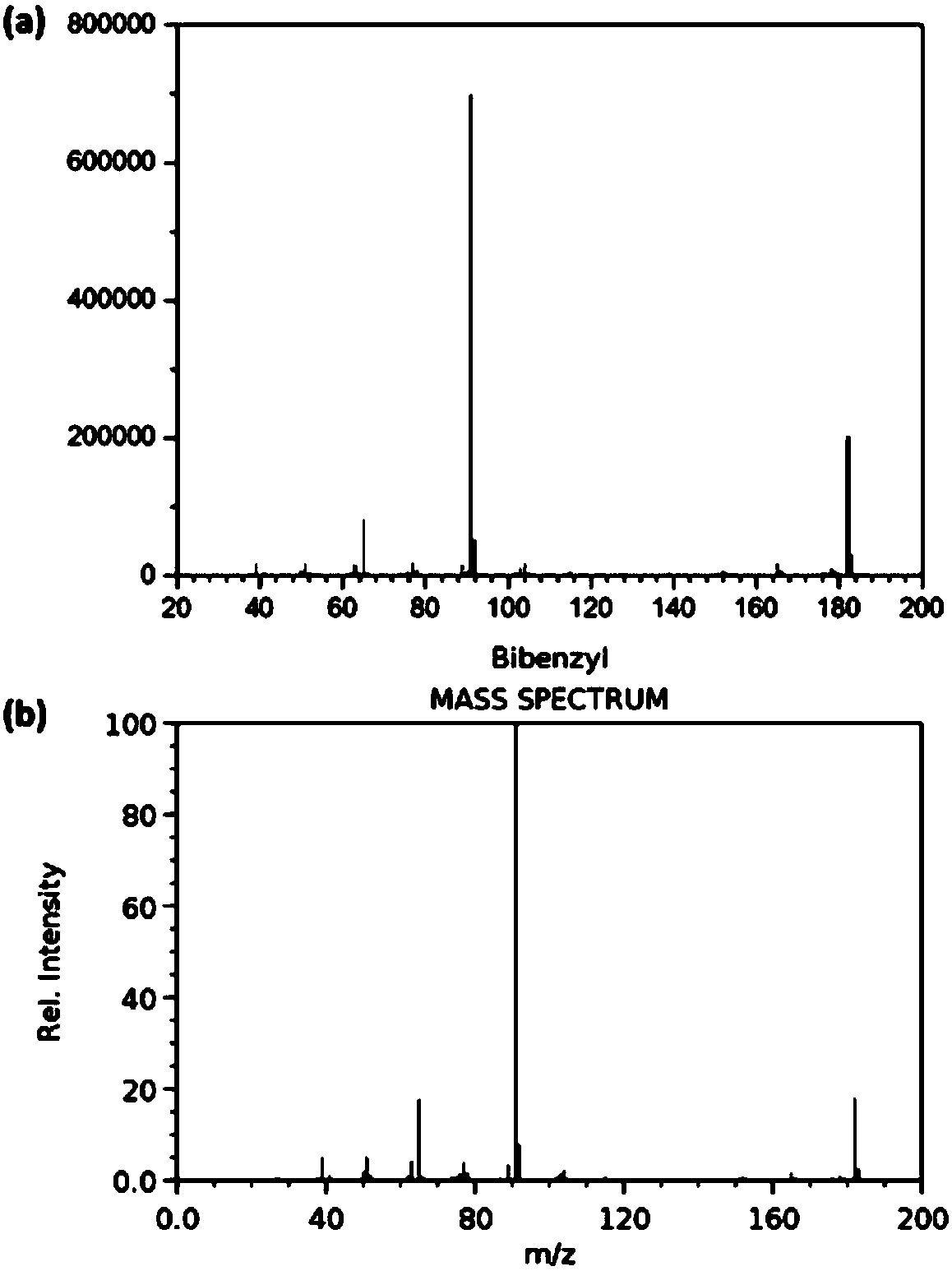
[0039] In a 5ml quartz glass reaction tube, add 1.0ml toluene and weigh 10mg ZnIn 2 S 4 To catalyze the reaction, replace the reaction tube with argon gas and seal it. The radiant flux at room temperature is 1.8W LED (450~460nm) for 12h. After the reaction, the product is detected by chromatography. The product mass spectrum and standard mass spectrum Figure one To. After the reaction, toluene is distilled out, and the toluene is recycled. The yield of 1,2-diphenylethane is 0.75g (g catalyst) -1 , The selectivity is 85%. The catalyst preparation steps are the same as in Example 2, and the raw material components are shown in Table 1.
Embodiment 2
[0041] In a 5ml quartz glass reaction tube, add 1.0ml toluene and weigh 10mg Ru-ZnIn 2 S 4 -0.5 Catalyze the reaction, replace the reaction tube with argon and seal it, and the radiant flux of 1.8W LED (450~460nm) at room temperature is illuminated for 12h. After the reaction is over, the product is detected by chromatography. The product mass spectrum and standard mass spectrum Figure one To. After the reaction, toluene is distilled out, and the toluene is recycled. The yield of 1,2-diphenylethane is 2.16g (g catalyst) -1 , The selectivity is 80%.
[0042] Ru-ZnIn 2 S 4 -0.5 catalyst is prepared by one-step hydrothermal method. 294mg zinc sulfate heptahydrate, 624mg indium trichloride tetrahydrate, 260mg cetylammonium bromide and 0.538mg RuCl 3 Add to 20mL of water; add 605mg of thioacetamide after stirring for half an hour; continue stirring for half an hour and then hydrothermally heat at 160°C for 20 hours. After cooling to room temperature, the crude Ru-ZnIn 2 S 4 -0.5 Th...
Embodiment 3
[0044] In a 100ml quartz glass reaction flask, add 20ml toluene and weigh 200mg Ru-ZnIn 2 S 4 -0.5 Catalyze the reaction, replace the reaction flask with argon and seal it, normal temperature xenon lamp (> 420nm, radiant flux 28.8W) illumination for 8h, after the reaction, the product is detected by chromatography, the product mass spectrum and standard mass spectrum Figure one To. After the reaction, toluene is distilled out, and the toluene is recycled. The yield of 1,2-diphenylethane is 15.9g (g catalyst) -1 , The selectivity is 74%. The catalyst preparation steps are the same as in Example 2, and the raw material components are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com