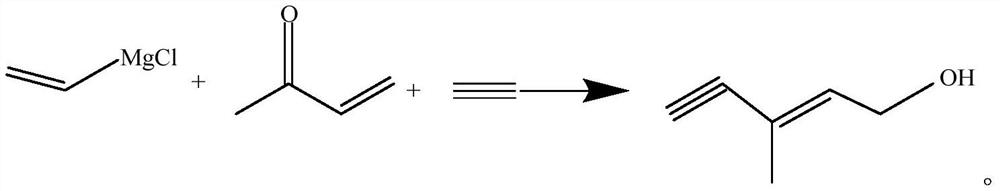
The preparation method of 3-methyl-2-penten-4-yn-1-ol
A technology of methyl ketene and pentene, which is applied in the field of preparation of 3-methyl-2-pentene-4-yn-1-ol, can solve the problems of complex reaction process, complicated process, safety risks and the like, and achieves the The purification steps are simple, the safety risks are reduced, and the side effects are less
Active Publication Date: 2022-05-31
ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY +1
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Extract with chloroform, wash with saturated sodium bicarbonate solution until neutral, recover chloroform, carry out crude distillation, and then distill, collect 60-65°C (1.06kPa) fractions to obtain fine six-carbon alcohol; it uses acetylene and calcium to prepare Calcium, the reaction process is complicated, the process is complicated, and the yield is low
[0004] Chinese patent CN104744211A discloses a kind of preparation method of acetylenic alcohol, the Grignard reagent of halogenated alkanes or halogenated alkenes is exchanged with acetylene to form bilateral acetylides, and reacts with acetylene again under pressure (0.3-1.5MPa) to obtain One-sided acetylide, followed by reaction with methyl ketene, followed by transposition with dilute sulfuric acid, yields 3-methyl-1-penten-4-yn-3-ol (hexacarbon alcohol), which requires pressure Reactor, and it takes two steps to obtain the unilateral magnesium alkyne compound, that is, first obtain the bilateral magnesium alkyne compound, then react with acetylene at low temperature under pressure to obtain the unilateral magnesium alkyne compound, and then maintain a certain pressure with methyl ketene Reaction to obtain six-carbon alcohol; it requires multi-step reaction, and requires a pressure reactor, the operation is complicated, and there are certain safety risks
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a preparation method of 3-methyl-2-pentene-4-yn-1-alcohol. In the existing methods, most of the reaction processes are complex, with many side reactions and low yields. The technical scheme adopted in the present invention is: first prepare the magnesium chloride ethylene Grignard reagent, and the magnesium chloride ethylene Grignard reagent forms a unilateral magnesium alkyne Grignard reagent with acetylene under normal pressure, and then condenses with methyl ketene, This is then transposed to give 3-methyl-2-pentene-4-yn-1-alcohol. The invention forms magnesium alkyne Grignard reagent under normal pressure, has few side reactions, mild process conditions, high efficiency, simple refining steps and high product yield.
Description
The preparation method of 3-methyl-2-pentene-4-alkyne-1-alcohol technical field The invention belongs to the preparation field of vitamin A and astaxanthin key intermediate, specifically a kind of 3-methyl-2- The preparation method of pentene-4-alkyne-1-alcohol. Background technique 3-methyl-2-pentene-4-alkyne-1-alcohol is the key intermediate for preparing vitamin A and astaxanthin, and has cis and trans Two isomers, both oily liquids; cis boiling point 65°C (1.2kPa), refractive index 1.4820; trans boiling point 73°C (1.2kPa), refractive index 1.4934. The general preparation method of 3-methyl-2-pentene-4-alkyne-1-alcohol is: first pass liquefied ammonia into the reaction chamber containing high iron nitrate. In the reaction tank, at below ‑40℃, add metal calcium to pass through acetylene within about 2h, after generating acetylene calcium, add methyl ketene dropwise, keep at ‑ Below 40 °C, stir for about 2 hours; then add ammonium chloride and stir for about 1 ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C33/048C07C29/40C07F3/02
Inventor 陈子杰吴志刚沈大冬陈浙蓉孙斌
Owner ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com