Preparation method of high purity 2-benzyloxy bromoethane
A benzyloxyethanol, high-purity technology is applied in the field of preparation of umeclidinium bromide intermediate 2-benzyloxybromoethane, can solve the problems of harsh reaction conditions, low yield, difficult purification and the like, and achieves short steps , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of crude product of 2-benzyloxy ethyl p-toluenesulfonate
[0037] 30.44g 2-benzyloxyethanol (0.2mol), 26.37gN,N-diisopropylethylamine (0.204mol), 1.22gN,N-dimethylaminopyridine (0.01mol), 38.89g p-toluenesulfonyl chloride ( 0.204mol) and 152mL of chloroform were sequentially added to a 500mL four-neck flask, kept at 20°C-30°C for 5h, TLC monitored the completion of the reaction, the reaction solution was washed with 0.1% malic acid aqueous solution, 0.1% sodium bicarbonate aqueous solution, distilled water After washing, the reaction solution was evaporated to dryness to obtain 60.0 g of yellow semi-solid oil (0.196 mol), yield 98.0%; HPLC purity 85.2%.
Embodiment 2
[0039] Purification of crude product of ethyl 2-benzyloxy p-toluenesulfonate
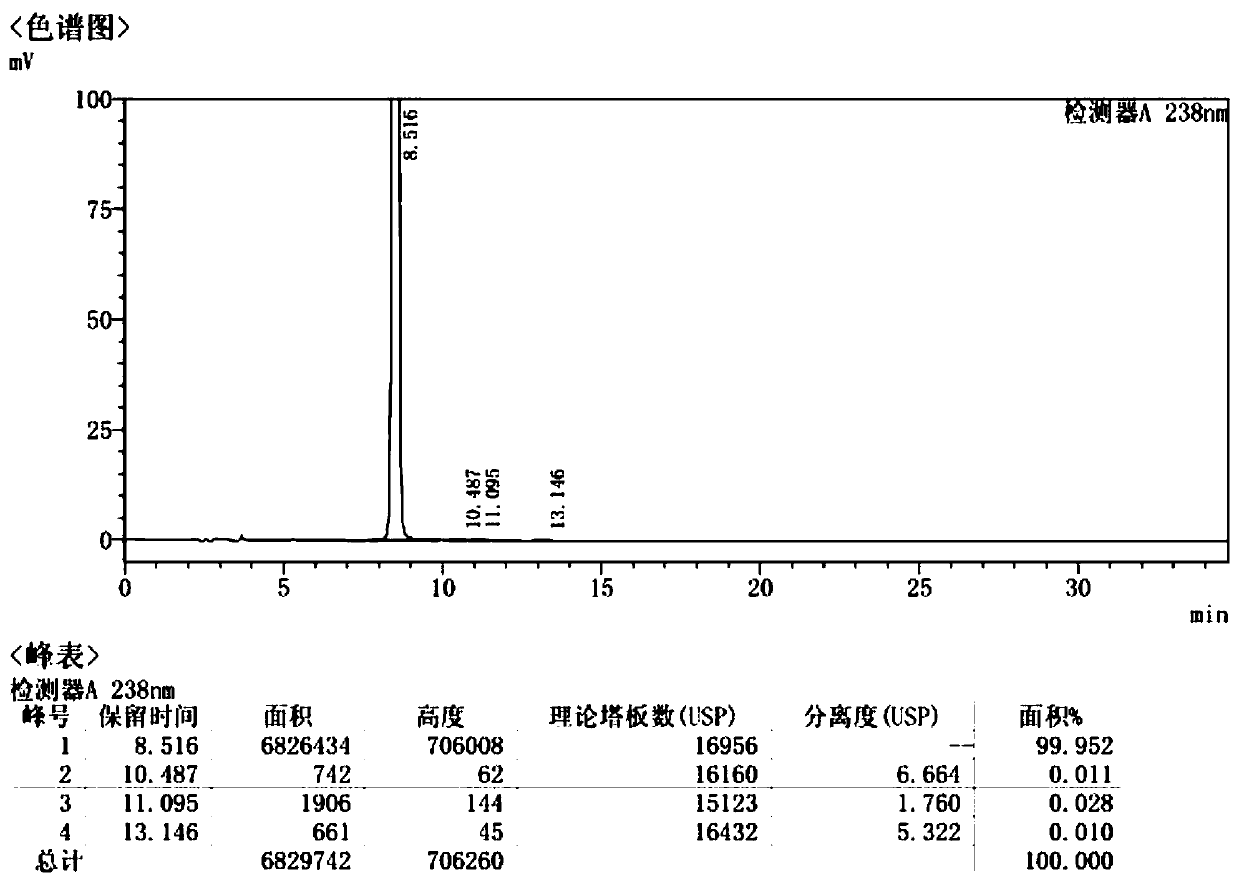
[0040] Add 30mL of absolute ethanol to 60.0g of 2-benzyloxy ethyl p-toluenesulfonate, heat until dissolved, add 60mL of petroleum ether dropwise, keep stirring at 30-50°C for 0.5-1h, cool down to 10°C, suction filter, filter cake Wash with 10 mL of petroleum ether to obtain 54.2 g of white crystalline powder, yield 90.3%; HPLC purity 98.6%.
Embodiment 3
[0042] Preparation of 2-Benzyloxybromoethane
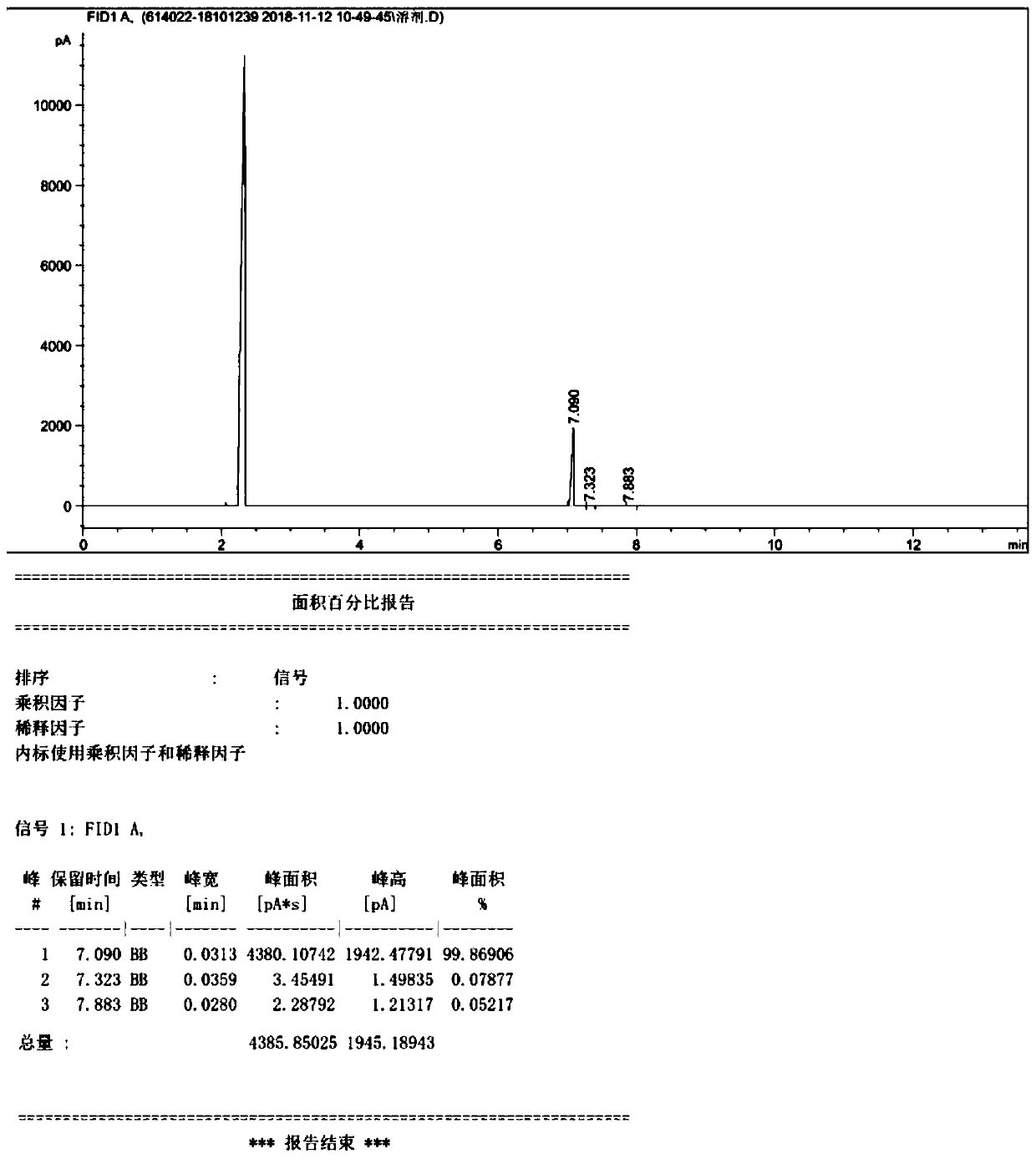
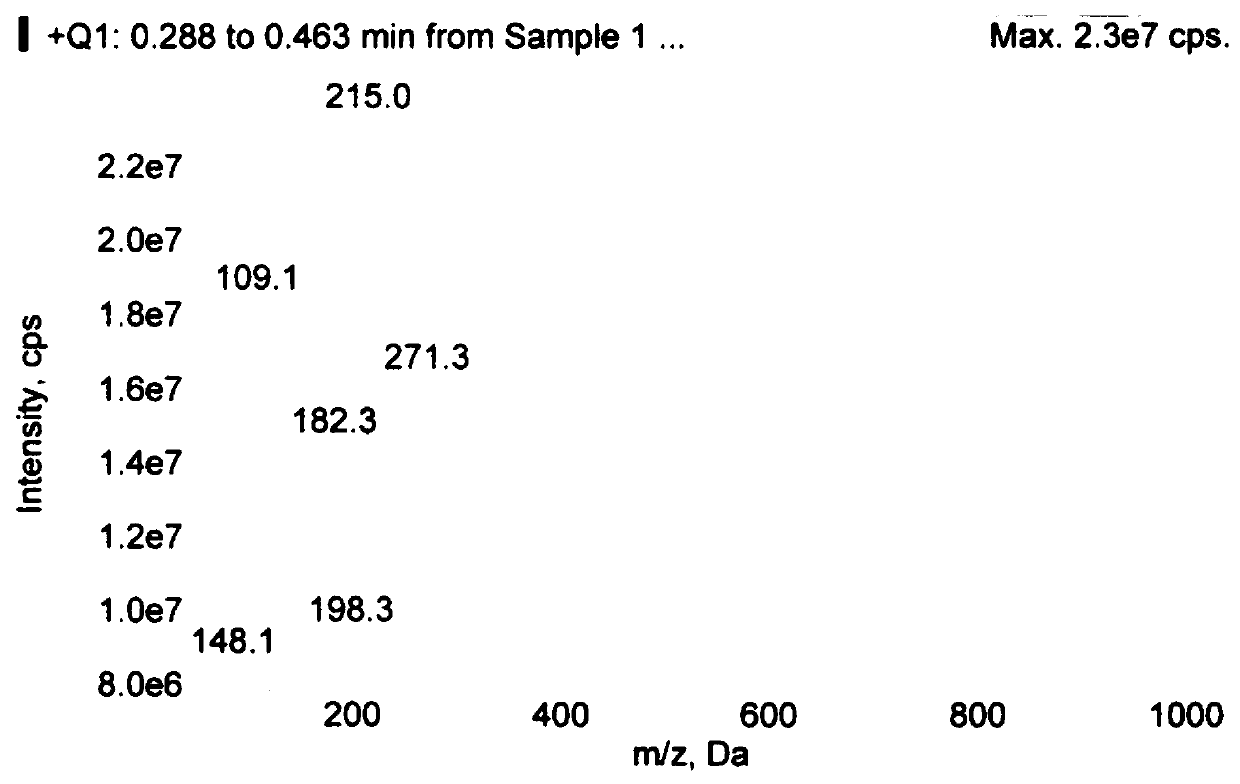
[0043] Add 54.2g of refined ethyl 2-benzyloxy-p-toluenesulfonate (0.1769mol), 23.05g of lithium bromide (0.2654mol), and 271mL of acetone into a 1000mL four-neck flask, keep warm at 20°C-30°C for 5h, TLC Monitor the completion of the reaction, add 300mL of water, extract the reaction solution 3 times with 200mL of ethyl acetate, combine the organic phases, wash once with 200mL of water, and evaporate the organic phase to obtain 35.6g of colorless and transparent oily substance, yield 93.5%; HPLC purity 98.5% %, GC purity 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com