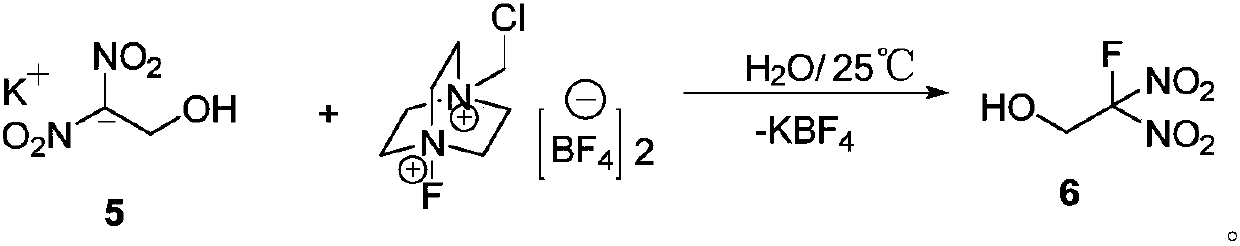
Preparation method of 2-fluoro-2, 2-dinitroethanol
A technology of dinitroethanol and dinitroacetylurea, which is applied in the field of energetic material preparation, can solve problems such as inability to apply engineering, achieve cheap and easy-to-obtain experimental operations, overcome expensive raw materials, and achieve high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of dinitromethane methane salt
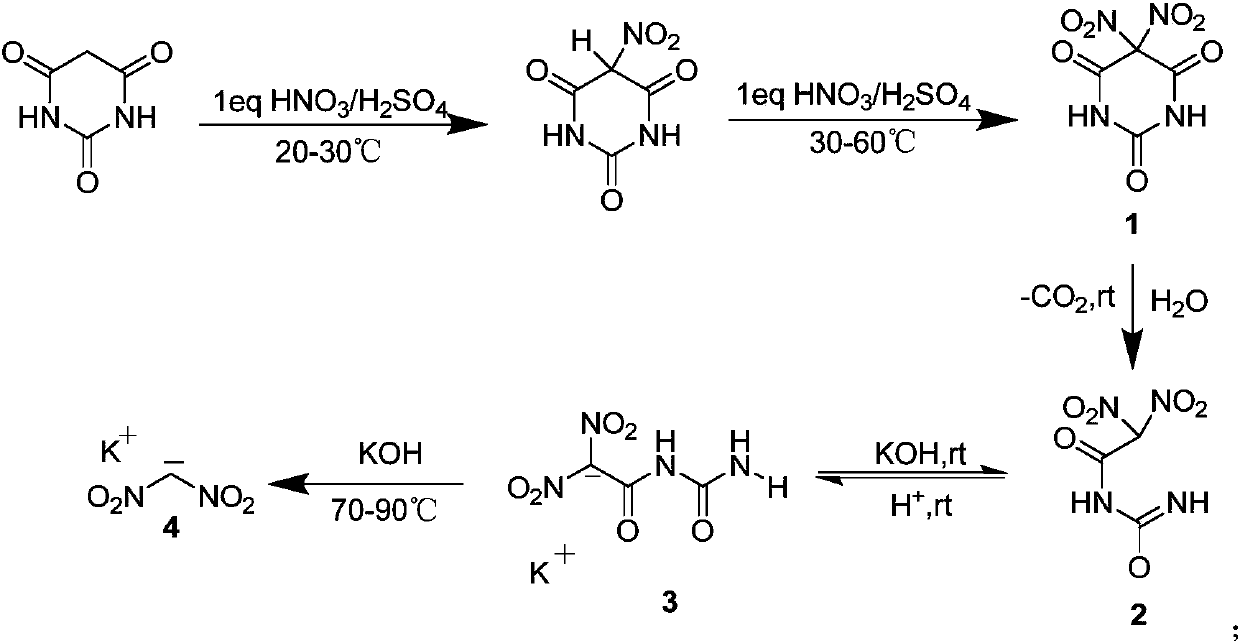
[0036] Measure 60ml of 95%-98% concentrated sulfuric acid and add it to a 250mL three-neck flask with a thermometer, control the temperature at 0-10°C, add 12.8g of barbituric acid in batches, and wait for the barbituric acid Add 10 mL of fuming nitric acid when it is almost dissolved, control the temperature below 25°C, slowly add it dropwise to the above solution, after the drop is complete, heat up to 45°C, the solution is white viscous liquid, continue to stir for 4 hours, there is a large amount of white powder After the reaction, it was cooled, filtered with suction, washed with trifluoroacetic acid (2 mL×5), and placed in a fume hood to air-dry overnight to obtain 20.60 g of a white powder solid with a yield of 92%.
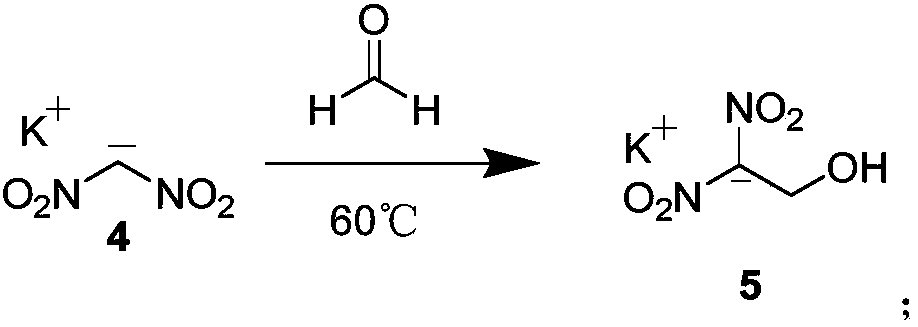
[0037] Add 5g of compound 1 to a 100mL monoclinic flask, add 10mL of water, and stir at room temperature for 2 hours. During this period, a large number of bubbles are found, the solution is yellow, a...
Embodiment 2
[0046] (1) Preparation of dinitromethane methane salt
[0047] Measure 60mL of 95%-98% concentrated sulfuric acid and add it to a 250ml three-neck flask with a thermometer, control the temperature at 0-10°C, add 12.8g of barbituric acid in batches, and wait for the barbituric acid Add 10 ml of fuming nitric acid when it is almost dissolved, control the temperature below 25°C, slowly add it dropwise to the above solution, after the drop is complete, heat up to 45°C, the solution is white viscous liquid, continue to stir for 4 hours, there is a large amount of white powder After the reaction, it was cooled, filtered with suction, washed with trifluoroacetic acid (2 mL×5), and placed in a fume hood to air-dry overnight to obtain 20.60 g of a white powder solid with a yield of 92%.
[0048] Add 5g of compound 1 to a 100mL monoclinic flask, add 15mL of water, and stir at room temperature for 2 hours. During this period, a large amount of bubbles are found, the solution is yellow, a...
Embodiment 3
[0056] (1) Preparation of dinitromethane methane salt
[0057] Measure 60mL of 95%-98% concentrated sulfuric acid and add it to a 250mL three-neck flask with a thermometer, control the temperature at 0-10°C, add 12.8g of barbituric acid in batches, and wait for the barbituric acid Add 10 mL of fuming nitric acid when it is almost dissolved, control the temperature below 25°C, slowly add it dropwise to the above solution, after the drop is complete, heat up to 45°C, the solution is white viscous liquid, continue to stir for 4 hours, there is a large amount of white powder After the reaction, it was cooled, filtered with suction, washed with trifluoroacetic acid (2 mL×5), and placed in a fume hood to air-dry overnight to obtain 20.60 g of a white powder solid with a yield of 92%.
[0058] Add 5g of compound 1 to a 100mL monoclinic flask, add 5ml of water, and stir at room temperature for 2 hours. During this period, a large number of bubbles are found, the solution is yellow, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com