Nano antibody and preparation method thereof
A nanobody and antibody technology, applied in the direction of antibodies, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of toxic and side effects of anti-PCSK9 protein monoclonal antibodies, and achieve low immunogenicity and high water solubility. , the effect of high antigen binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 PCSK9 nanobody phage display library construction;
[0100] (1) PCSK9 immune camel
[0101] Mix 1mg of PCSK9 with an equal volume of Freund's adjuvant to 5ml, inject it at 3-5 points under the skin of the camel's neck, and collect blood from the ear vein of the camel before immunization. Immunize once a month, and immunize 4 times in total; take 10ml of peripheral blood of camels for each immunization. Fix the camel's head to one side when collecting blood, shave (pluck) the skin of the animal's blood collection site first, disinfect with 75% alcohol, collect blood after drying, and press the jugular vein groove with your fingers. The site was sterilized and the blood was drawn through the needle, and 10ml of blood was collected in a 15ml EDTA anticoagulant tube, which was shaken continuously and slowly, mixed thoroughly, placed on ice, and transported back to the laboratory.
[0102] (2) Separation of blood lymphocyte samples
[0103] Lymphocytes were sepa...
Embodiment 2
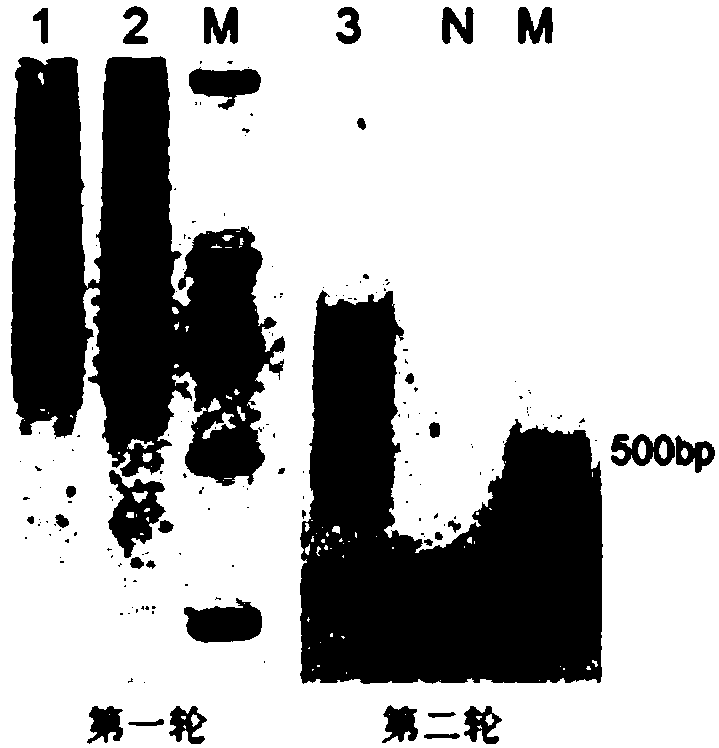
[0141] Example 2 Elutriation of PCSK9 Nanobody Antibody Using Phage Display Technology
[0142] (1) Affinity PCSK9 nanobody phage library panning
[0143] Take 100ng PCSK9 antigen coated ELISA plate, 4 ℃, overnight incubation. The next day, add the rescued PCSK9 nanobody phage, at room temperature, and incubate for 2 hours; wash the wells 10 times with PBST, add 100 μl triethylamine, room temperature, and incubate for 30 minutes. The collected phages are the PCSK9 nanobody phage library obtained by affinity panning; Take 10 μl of infected TG cells to coat the plate for determining the number of clones after screening, and the remaining screened phages are used for amplification.
[0144] (2) Amplification and rescue of phage after screening
[0145] The amplification and rescue method is the same as in Example 1 (7). The obtained PBS suspension is the amplified phage after the first round of screening, stored at 4°C, and used for the next round of screening; according to the...
Embodiment 3
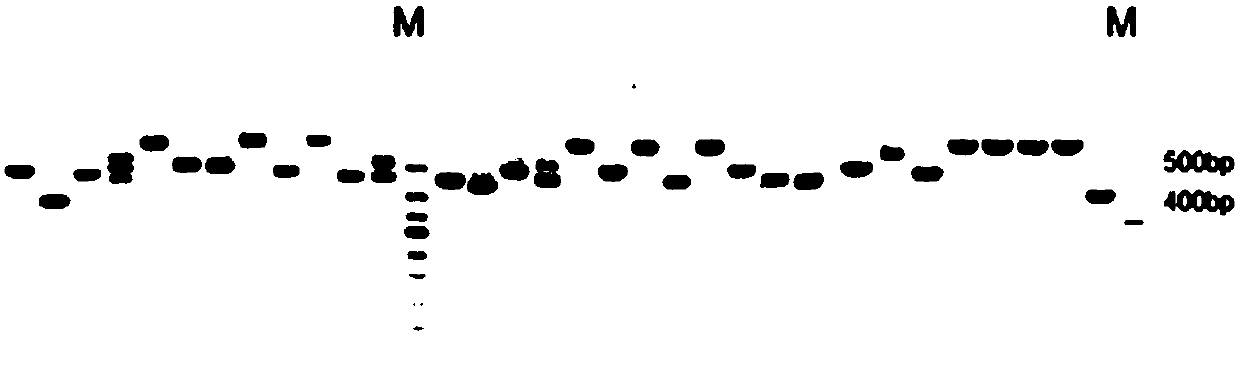
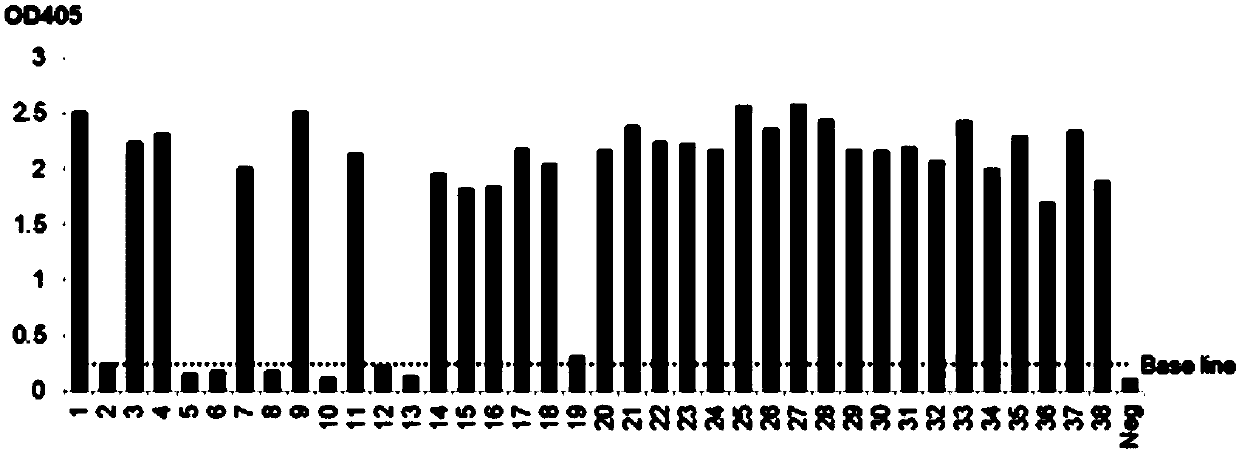
[0164] Example 3 Induced Expression and Purification of PCSK9 Nanobody
[0165] (1) Construction of PCSK9 Nanobody Expression Bacteria
[0166] First, culture the PCSK9 nanobody monoclonal transfer medium at 37°C overnight; the next day, use Plasmid Mini Kit I (OMEGA) was used to extract the plasmid, and after agarose gel electrophoresis and concentration determination, the plasmid containing the PCSK9 nanobody sequence was transformed into expression bacteria HB2151, and the plate was spread and incubated overnight at 37°C.
[0167] (2) Induced expression of PCSK9 nanobody
[0168] The next day, pick 5 clones from the plate for clone PCR to verify whether the plasmid is transferred into the expression strain; pick positive clones and culture them at 37°C until OD 600 0.6-0.8, add IPTG to induce expression. Centrifuge the bacterial liquid, collect the bacterial pellet, resuspend the pellet with lysis buffer, disrupt the bacterial cells by ultrasonic, and collect the supern...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com