CuBr2@Zr-MOF catalyst as well as preparation method and application thereof
A catalyst and reaction technology, used in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., which can solve the problem of little research on "click" reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
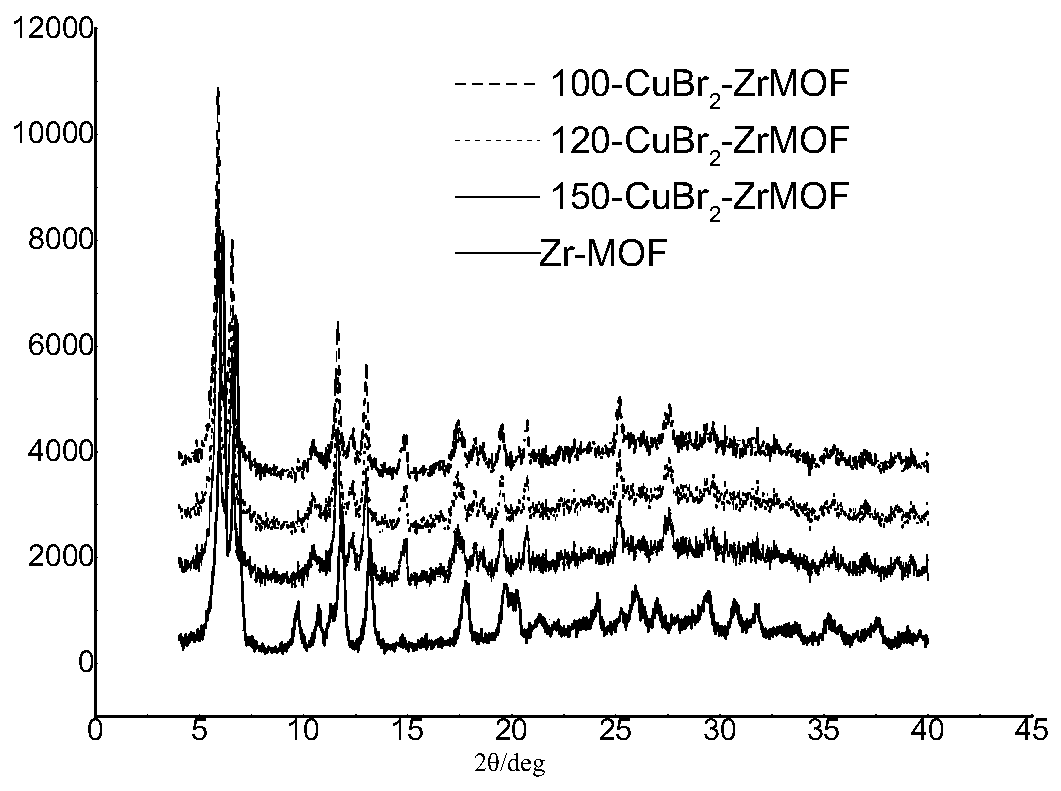
[0031] Accurately weigh ZrCl into a 100mL pressure-resistant tube 4 (212mg), 2,2′,5,5′-dipyridinedicarboxylic acid (H 2 bpydc) (220 mg) was dissolved in 40 mL of DMF, stirred at room temperature for 5 minutes, added 10 mL of glacial acetic acid to the system, ultrasonicated for 20 minutes, and the uniformly dispersed system was heated in an oven at 120°C, taken out after 48 hours, and cooled to At room temperature, a white powdery solid was obtained. The product was washed with DMF, water and acetone three times in sequence, and then dried in vacuum at 100°C to obtain Zr-MOF with a yield of about 60%. Characterized by PXRD, the results are shown in figure 1 .
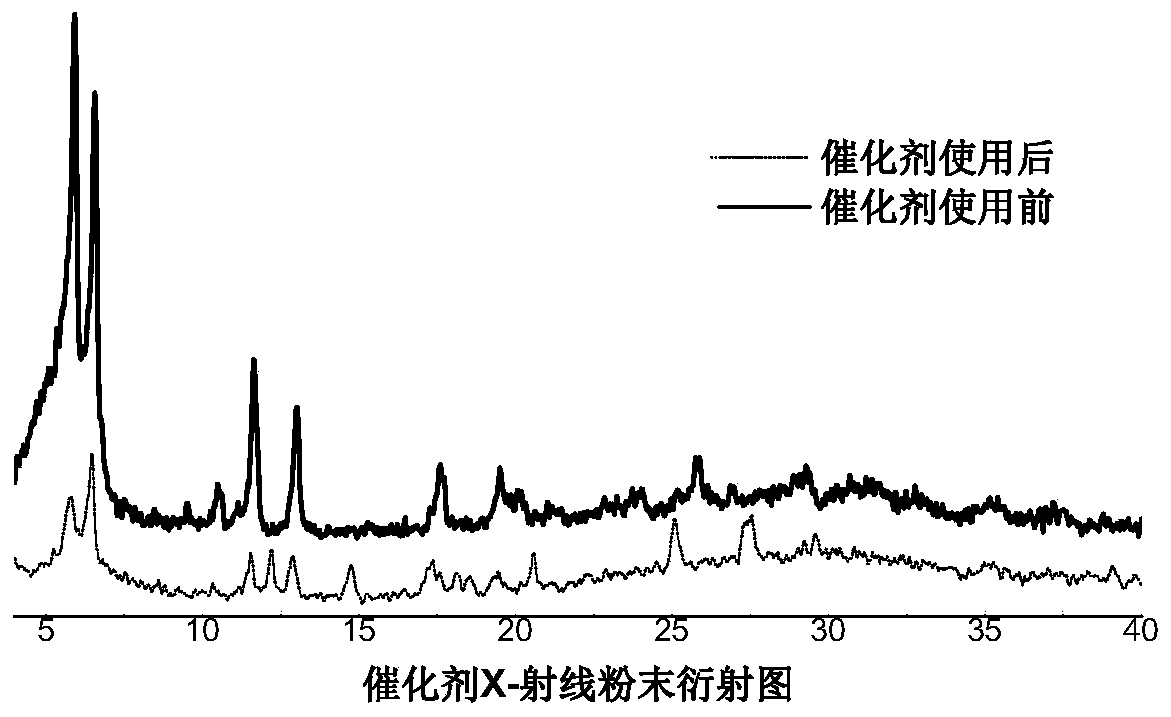
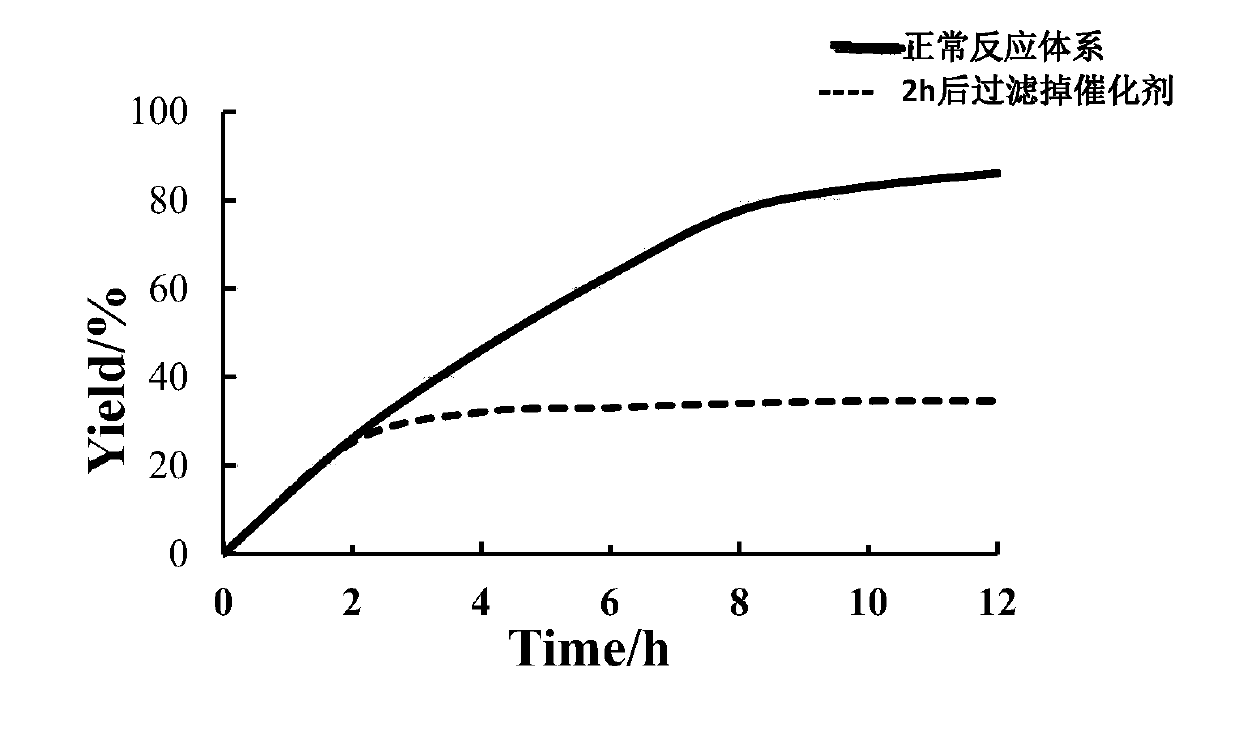
[0032] Weigh Zr-MOF (100 mg), CuBr 2 (65mg) was added into 15mL water, and the mixture was stirred at room temperature for 4 hours to obtain a green powder, which was washed with water and acetone three times in sequence, and dried in vacuum at 100°C. The product was collected for PXRD characterization and ICP test. ...
Embodiment 2
[0034] Accurately weigh ZrCl into a 100mL pressure-resistant tube 4 (212mg), 2,2′,5,5′-dipyridinedicarboxylic acid (H 2 bpydc) (210mg) was dissolved in 40mL DMF, stirred at room temperature for 5 minutes, 10mL glacial acetic acid was added to the system, ultrasonicated for 20 minutes, the uniformly dispersed system was heated in an oven at 100°C, taken out after 48 hours, and cooled to At room temperature, a white powdery solid was obtained. The product was washed with DMF, water, and acetone three times in sequence, and dried in vacuum at 100° C. to obtain Zr-MOF with a yield of about 50%.
[0035] Weigh Zr-MOF (100 mg), CuBr 2 (62 mg) was added into 15 mL of water, and the mixture was stirred at room temperature for 4 hours to obtain a green powder, which was washed with water and acetone three times in turn, and dried in vacuum at 105°C. The product was collected for PXRD characterization and ICP test. The results are shown in figure 1 .
Embodiment 3
[0037] Accurately weigh ZrCl into a 100mL pressure-resistant tube 4 (212mg), 2,2′,5,5′-dipyridinedicarboxylic acid (H 2 bpydc) (200mg) was dissolved in 40mL DMF, stirred at room temperature for 5 minutes, added 10mL glacial acetic acid to the system, ultrasonicated for 20 minutes, put the uniformly dispersed system in a 150°C oven to heat, took it out after 48 hours, and cooled to At room temperature, a white powdery solid was obtained. The product was washed with DMF, water and acetone three times in sequence, and dried in vacuum at 110°C to obtain Zr-MOF with a yield of about 45%.
[0038] Weigh Zr-MOF (100 mg), CuBr 2 (60mg) was added into 15mL water, and the mixture was stirred at room temperature for 4 hours to obtain a green powder, which was washed with water and acetone three times in turn, and dried in vacuum at 105°C. The product was collected for PXRD characterization and ICP test. The results are shown in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com