Metal halide acid radical ion liquid for catalyzing acetylene hydrochlorination as well as application method thereof
A technology of acetylene hydrochlorination and ionic liquids, applied in the field of metal halide ionic liquids, can solve the problems of unfavorable industrial production, low catalytic activity, poor stability, etc., to avoid local overheating, catalytic activity and selectivity reduction, thermal stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
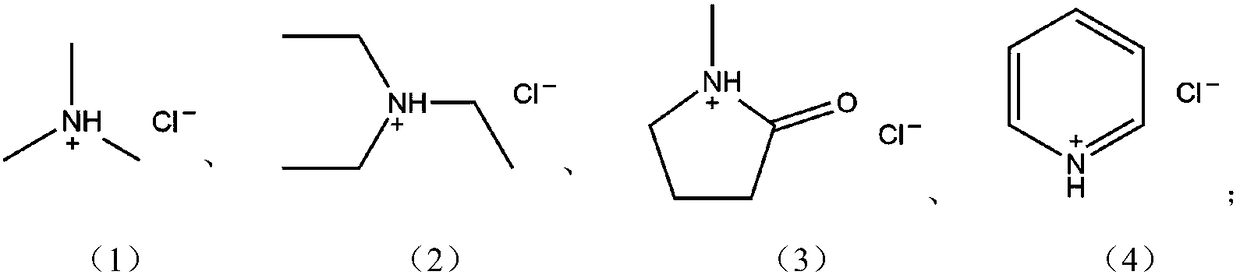
[0029] (1) Slowly add 0.25 moles (24.75 grams) of CuCl to 0.25 moles (23.89 grams) of trimethylamine hydrochloride, under nitrogen protection, stir at 100°C for 12 hours to complete the reaction to obtain trimethylammonium chloride Cuprite ionic liquid [Me 3 NH]Cl / CuCl.
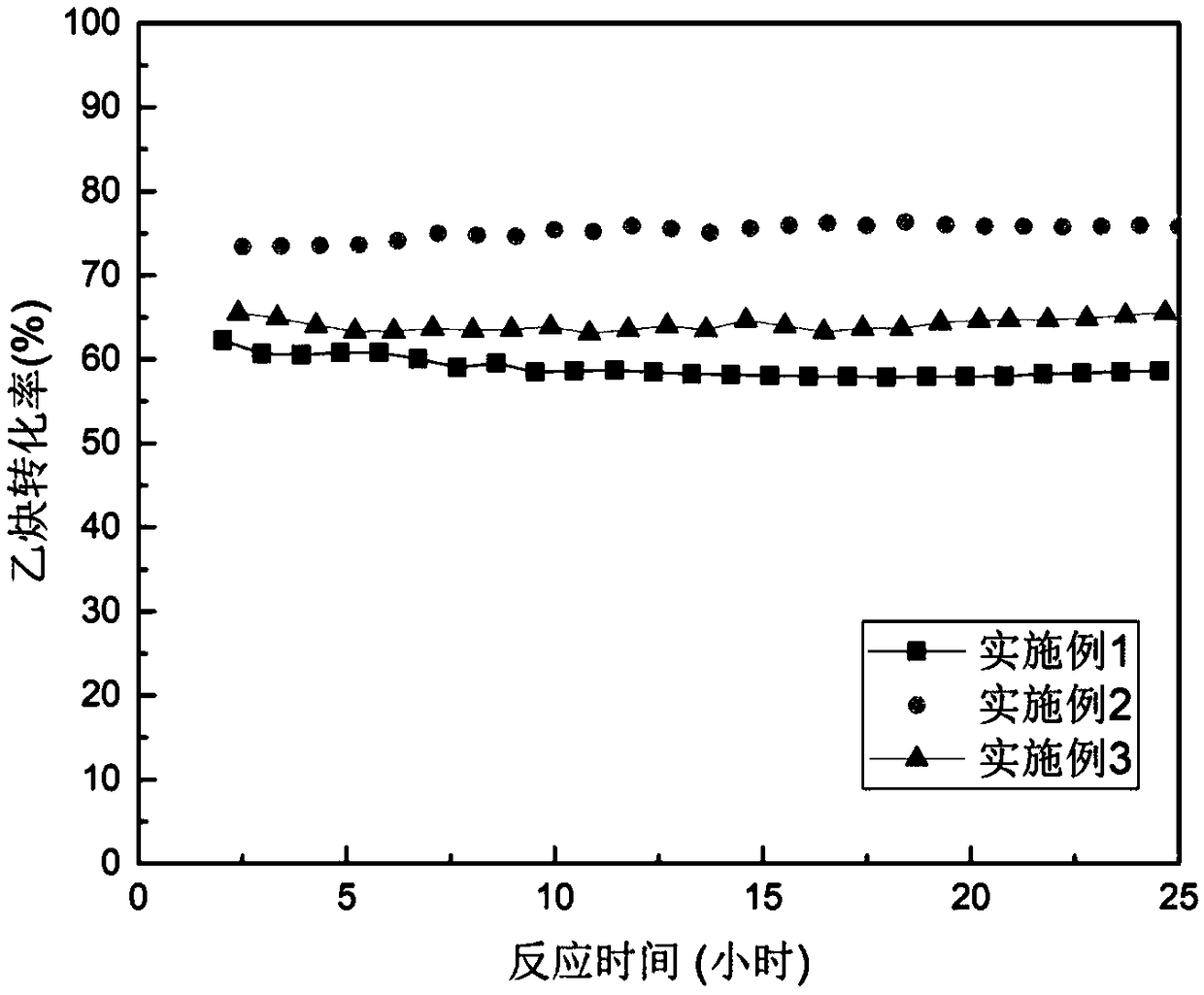
[0030] (2) get 18 milliliters of synthetic trimethylammonium cuprous chloride ionic liquid [Me 3 NH]Cl / CuCl, put it into the reaction tube for acetylene hydrochlorination reaction, the conversion rate of acetylene was 58.46% after reaction at 140°C for 12 hours, the selectivity of vinyl chloride was 99.69%, and the reaction continued for 24 hours There was no significant decrease in activity. The change curve of acetylene conversion rate with reaction time is shown in figure 1 .
Embodiment 2
[0032] (1) Slowly add 0.25 moles (24.75 grams) of CuCl to 0.25 moles (34.41 grams) of triethylamine hydrochloride, under nitrogen protection, stir at 100°C for 12 hours to complete the reaction to obtain triethylammonium chloride Cuprousite ionic liquid [Et 3 NH]Cl / CuCl.
[0033] (2) get 18 milliliters of synthetic triethylammonium cuprous chloride ionic liquid [Et 3 NH]Cl / CuCl, put it into the reaction tube for acetylene hydrochlorination reaction, the conversion rate of acetylene was 75.87% after reaction at 140°C for 12 hours, the selectivity of vinyl chloride was 99.78%, and the reaction continued for 24 hours There was no significant decrease in activity. The change curve of acetylene conversion rate with reaction time is shown in figure 1 .
Embodiment 3
[0035] (1) Slowly add 0.25 moles (24.75 grams) of CuCl to 0.25 moles (28.89 grams) of pyridine hydrochloride, under nitrogen protection, and stir at 100°C for 12 hours to complete the reaction to obtain pyridine cuprous chloride Ionic liquid [PyH]Cl / CuCl.
[0036] (2) Get 18 milliliters of synthetic pyridine cuprous chloride ionic liquid [PyH]Cl / CuCl, put it into the reaction tube and carry out the acetylene hydrochlorination reaction, and the acetylene conversion rate is 63.53%, the selectivity of vinyl chloride is 99.73%, and there is no obvious decrease in activity during the 24-hour continuous reaction. The change curve of acetylene conversion rate with reaction time is shown in figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com