Glucocorticoid drug tablet and preparation method thereof
A glucocorticoid and tablet technology, which is applied in the field of glucocorticoid drug tablets and its preparation, can solve the problems of affecting the dissolution rate, low production efficiency, and low yield of tablets, so as to improve the dissolution rate and production efficiency , The effect of reducing equipment energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Feeding according to 10000 pieces
[0034] Recipe: Methylprednisolone 40g
[0035] Lactose monohydrate 720g
[0036] Cornstarch 210g
[0037] Crospovidone 20g
[0039] Preparation Process:
[0040] 1) Raw material crushing: the crushing air flow is 1.0Mpa, the feed rate is 0.6kg / h, the raw material is crushed in batches to D90 and then averaged, and the average D90 is 30~40μm.
[0041] 2) Feed on the basis of preparing 10,000 tablets. The above-mentioned excipients in the prescription amount except crospovidone and magnesium stearate are passed through a 60-mesh sieve respectively, and mixed with the above-mentioned methylprednisolone powder. When mixing, a three-dimensional mixer is used. 10 rpm, mixing for 15 minutes.
[0042] 3) Add the prescribed amount of crospovidone, rotate at 10 rpm, and mix for 10 minutes.
[0043] 4) Add the prescribed amount of magnesium stearate, rotate at 10 rpm, mix for 10 minutes, and perform tablet ...
example 1
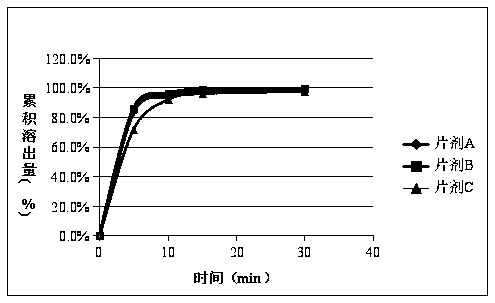
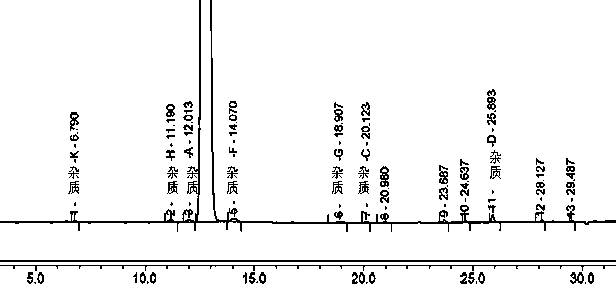
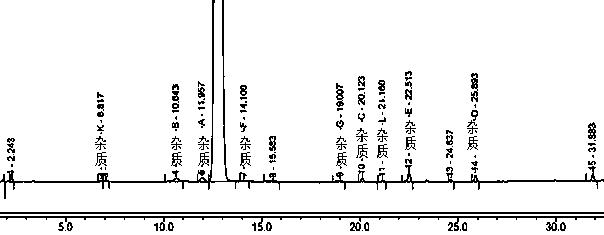
[0047] Example 1 Tablet A and the original research sample B have no significant increase in impurities after 30 days of high temperature, and the impurities in tablet C have increased significantly after being placed at high temperature for 30 days, especially impurity D. Therefore, the stability of methylprednisolone tablets prepared by the preparation process in this example is The sex is better, and the quality is consistent with the original research product. The data are shown in Tables 1, 2, and 3.
[0048] Table 1 Example 1 Tablet A high temperature storage 0 days and 30 days related substance results
[0049]
[0050] N.D. means not detected
[0051] Table 2 The results of related substances in the original research sample B placed at high temperature for 0 days and 30 days
[0052]
[0053] N.D. means not detected
[0054] Table 3 Results of related substances in Tablet C stored at high temperature for 0 days and 30 days
[0055]
[0056] N.D. means not ...
Embodiment 2
[0058] Feeding according to 10000 pieces
[0059] formula:
[0060] Methylprednisolone 40g
[0061] Lactose monohydrate 930g
[0062] Low-substituted hydroxypropyl cellulose 20g
[0064] Preparation Process:
[0065] 1) Raw material crushing: the crushing air flow is 1.0Mpa, the feed rate is 0.6kg / h, the raw material is crushed in batches to D90 and then averaged, and the average D90 is 30~40μm.
[0066] 2) Feed for the preparation of 10,000 tablets, pass the prescribed amount of lactose monohydrate through a 60-mesh sieve, and mix with the above-mentioned methylprednisolone powder. When mixing, use a three-dimensional mixer with a speed of 10 rpm and mix for 15 minutes.
[0067] 3) Add the prescribed amount of low-substituted hydroxypropyl cellulose, rotate at 10 rpm, and mix for 10 minutes.
[0068] 4) Add the prescribed amount of calcium stearate, rotate at 10 rpm, mix for 10 minutes, and perform tablet compression.
[0069] Get above-ment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com