Persulfate activating method
A persulfate and treatment method technology, applied in chemical instruments and methods, oxidized water/sewage treatment, water pollutants, etc., can solve problems such as non-recyclable, low cost, and potential toxicity, and improve the removal rate of wastewater , Strengthen the effect of governance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
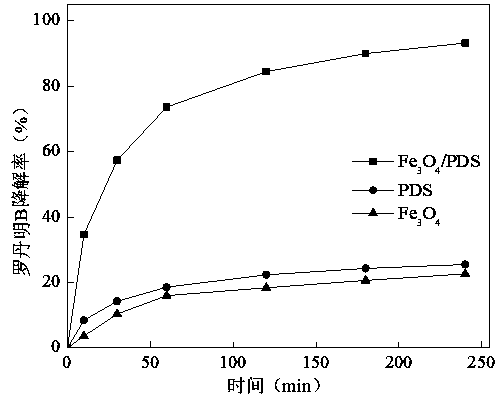
[0020] Experimental conditions: Experimental conditions: when adding PDS alone, the dosage of PDS is 3 g / L; adding Fe alone 3 o 4 when Fe 3 o 4 The dosage is 1 g / L; Fe 3 o 4 In the / PDS system, the dosage of PDS is 3 g / L, Fe 3 o 4 The dosage is 1 g / L, and the initial pH of the solution is 3.
[0021] Depend on figure 1 It can be seen that when PDS is added alone, the degradation rate of rhodamine B is 25.6%. PDS itself has a certain oxidation ability. When PDS is dissolved in aqueous solution, a hydrolysis reaction will occur to generate persulfate (S 2 o 8 2- ), its oxidation-reduction potential is 2.01V, which can effectively oxidize some pollutants, but its oxidation ability is relatively weak, so the degradation rate of rhodamine B in the reaction system is not high.
[0022] Dosing Fe alone 3 o 4 When , the degradation rate of rhodamine B in the system was only 22.7%. This is mainly because Fe 3 o 4 The crystal particles are larger and clustered, and have ...
Embodiment 2
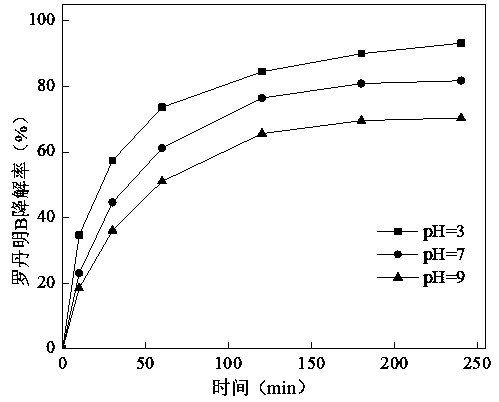
[0025] Experimental conditions: PDS dosage is 3 g / L, reaction temperature is 25°C, Fe 3 o 4 The dosage was 1.0 g / L, and the initial pH of the solution was 3, 7, and 9, respectively. The treatment effect of the system on rhodamine B dye wastewater was mainly investigated under acidic, neutral and alkaline conditions. Depend on figure 2 It can be seen that the degradation rate of rhodamine B is the best under acidic conditions, with a degradation rate of 93.2 %, followed by neutral conditions, with a removal rate of 81.8 %; the worst treatment effect is under alkaline conditions, with a degradation rate of 70.4 %.
Embodiment 3
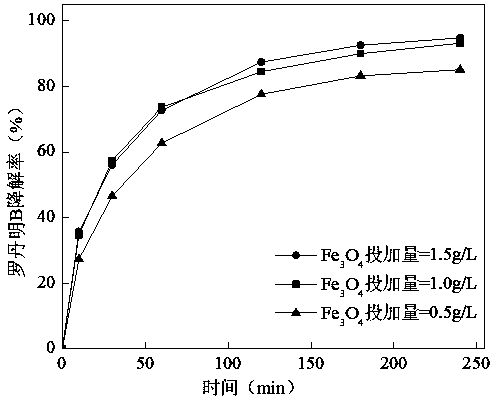
[0027] Experimental conditions: the initial pH of the solution is 3, the dosage of PDS is 3 g / L, the reaction temperature is 25 ℃, Fe 3 o 4 The dosage is 0.5, 1.0, 1.5 g / L respectively. Depend on image 3 It can be seen that when Fe 3 o 4 When the dosage is 0.5 g / L, the degradation rate of Rhodamine B is 85.2%, and when the dosage increases to 1.0 g / L, the removal rate is 93.2%. Ming B degradation rate. This is because Fe 3 o 4 The increase in the dosage is beneficial to improve the surface Fe 2+ The probability of exposure to persulfate, which promotes SO 4 - ·generate. But Fe 3 o 4 When the dosage was further increased to 1.5 g / L, the removal rate did not increase significantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com