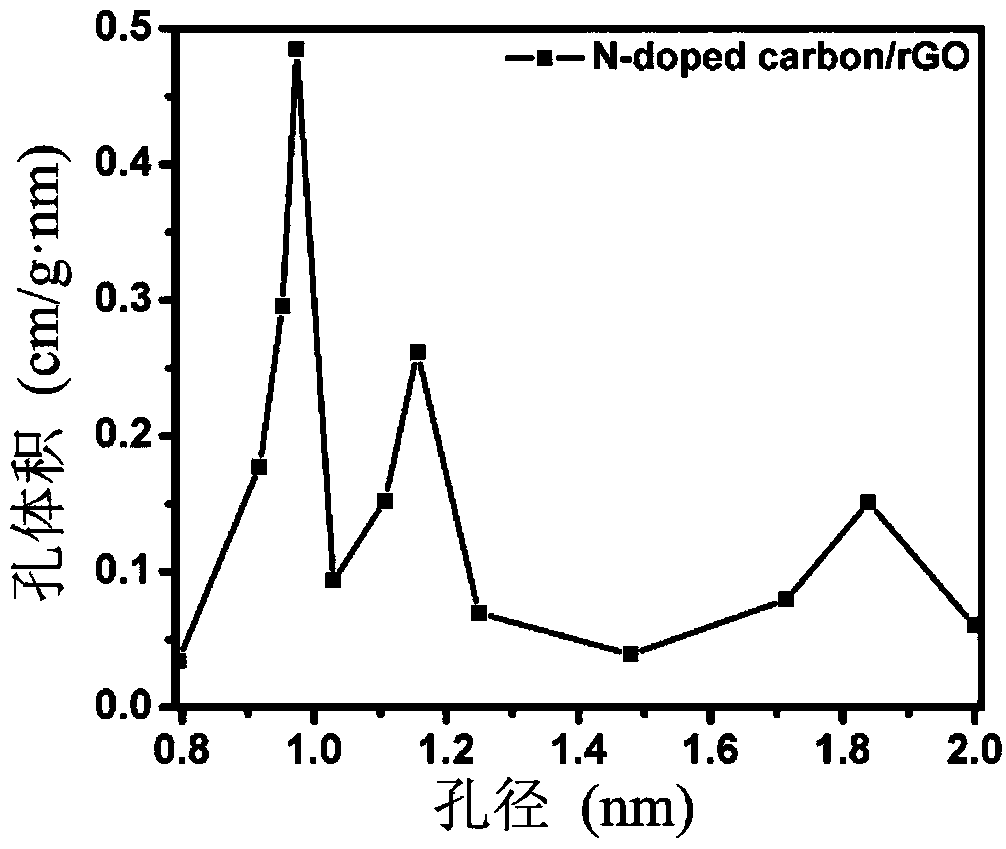
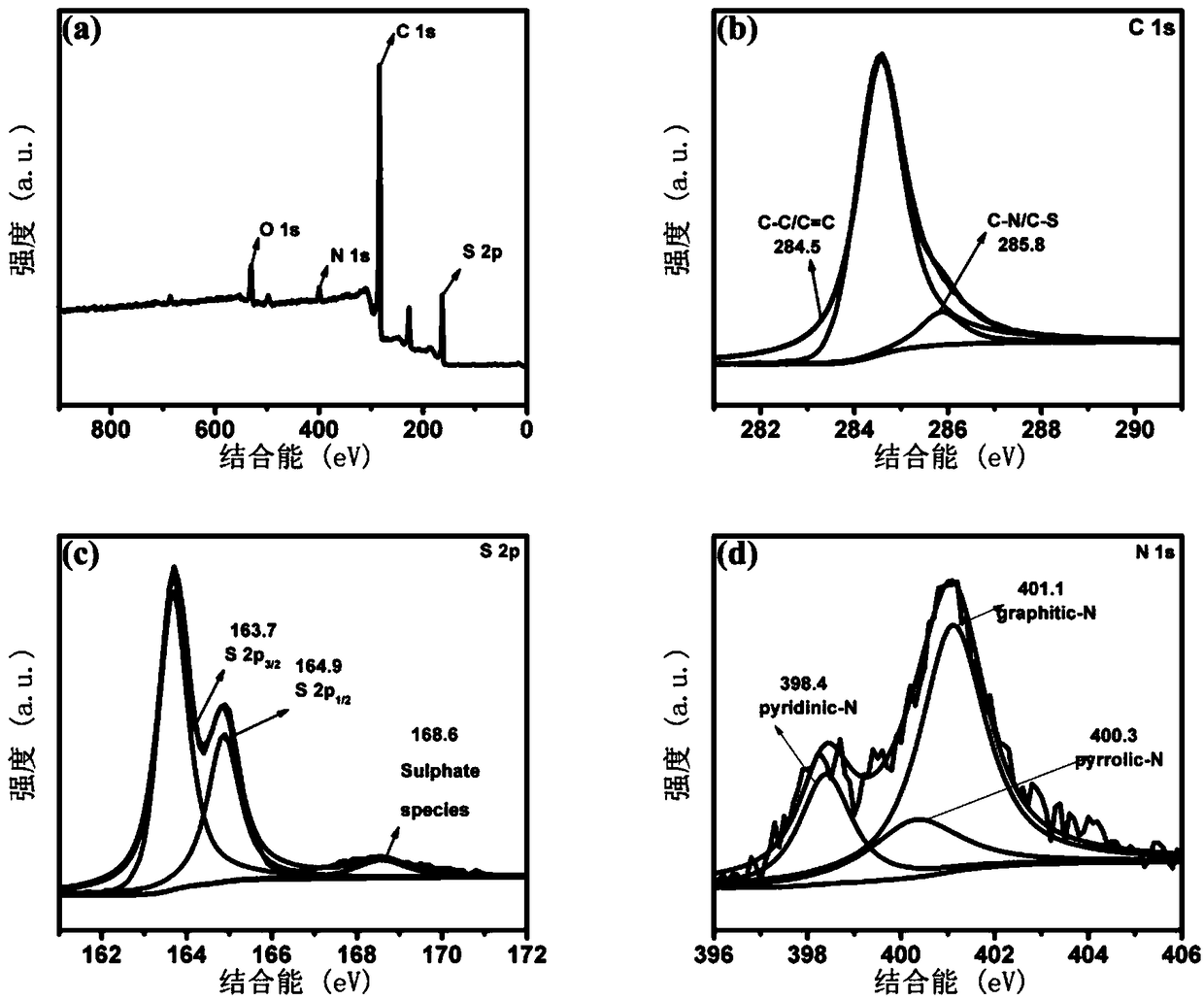
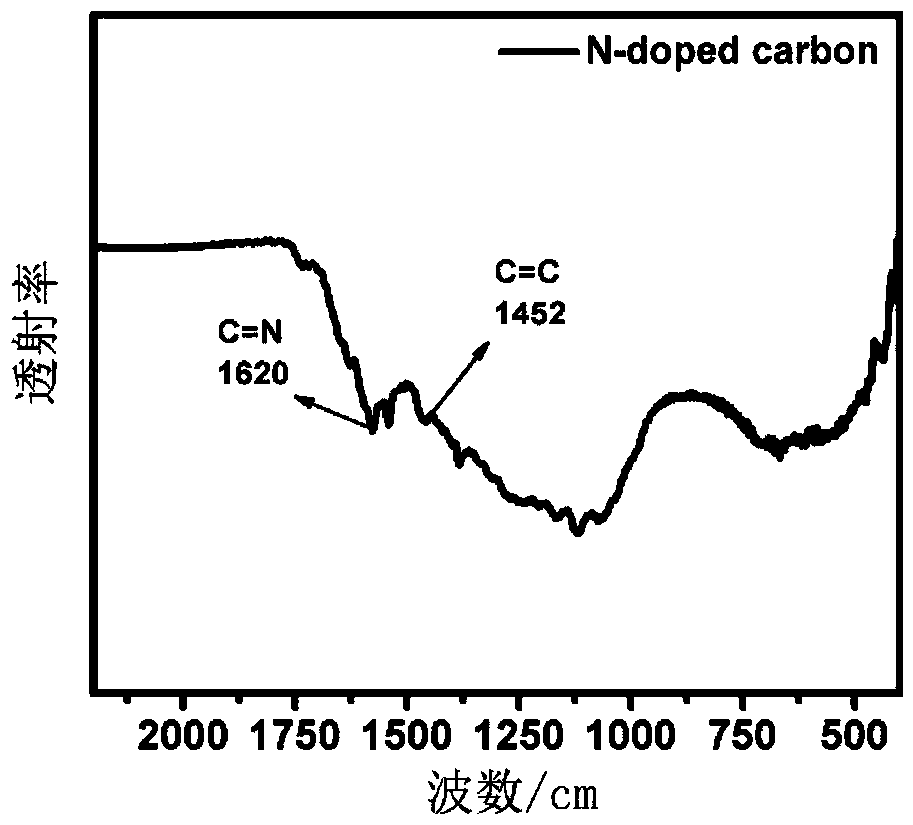
A preparation method of lithium sulfide battery cathode material
A cathode material, lithium-sulfur battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems affecting the wide application of lithium-sulfur batteries, difficult to control microscopic morphology, electrode material volume expansion, etc., to enhance electrochemical performance. , the effect of improving the reversibility of the reaction and improving the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1, improving the Hummers method to prepare graphene oxide:
[0041] 1g of graphite, 27ml of 95% H 2 SO 4 , 3ml concentration of 0.1M H 3 PO 4 Place in a three-necked flask, and add 6g of potassium permanganate in portions, stir in an ice-water bath for 1h, raise the temperature to 50°C, and keep the reaction for 12h. The resulting product was poured into ice water, and 30% hydrogen peroxide was added while stirring until the color of the solution turned golden yellow, then filtered, and the product was washed with 5% HCL and distilled water until the pH value was close to 7. The obtained graphite oxide is dispersed in water, ultrasonicated for 8 hours, and finally configured as a 2 mg / ml graphene oxide solution for later use;
[0042] Step 2: In situ polymerization of acrylonitrile / graphene oxide composites
[0043] PAN / GO composites were prepared by in situ polymerization. First, 125ml of deionized water, 12.5ml of acrylonitrile and 50ml of GO solution with ...
Embodiment 2
[0058] Step 1, improving the Hummers method to prepare graphene oxide:
[0059] 1g of graphite, 27ml of 95% H 2 SO 4 , 3ml concentration of 0.1M H 3 PO 4 Place in a three-necked flask, and add 6g of potassium permanganate in portions, stir in an ice-water bath for 1h, raise the temperature to 50°C, and keep the reaction for 12h. The resulting product was poured into ice water, and 30% hydrogen peroxide was added while stirring until the color of the solution turned golden yellow, then filtered, and the product was washed with 5% HCL and distilled water until the pH value was close to 7. The obtained graphite oxide is dispersed in water, ultrasonicated for 8 hours, and finally configured as a 2 mg / ml graphene oxide solution for later use;
[0060] Step 2: In situ polymerization of acrylonitrile / graphene oxide composites
[0061] PAN / GO composites were prepared by in situ polymerization. First, 125ml of deionized water, 12.5ml of acrylonitrile and 50ml of GO solution with ...
Embodiment 3
[0075] Step 1, improving the Hummers method to prepare graphene oxide:
[0076] 1g of graphite, 27ml of 95% H 2 SO 4 , 3ml concentration of 0.1M H 3 PO 4 Place in a three-necked flask, and add 6g of potassium permanganate in portions, stir in an ice-water bath for 1h, raise the temperature to 50°C, and keep the reaction for 12h. The resulting product was poured into ice water, and 30% hydrogen peroxide was added while stirring until the color of the solution turned golden yellow, then filtered, and the product was washed with 5% HCL and distilled water until the pH value was close to 7. The obtained graphite oxide is dispersed in water, ultrasonicated for 8 hours, and finally configured as a 2 mg / ml graphene oxide solution for later use;
[0077] Step 2: In situ polymerization of acrylonitrile / graphene oxide composites
[0078] PAN / GO composites were prepared by in situ polymerization. First, 125ml of deionized water, 12.5ml of acrylonitrile and 50ml of GO solution with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com