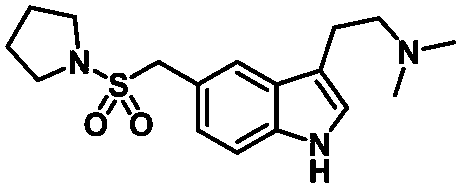
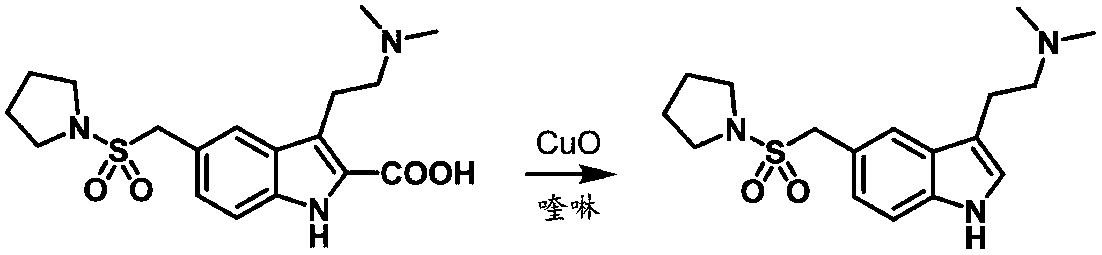
Preparation method of anti-migraine drug almotriptan
A technology for almotriptan and migraine, which is applied in the field of preparation of anti-migraine drug almotriptan, can solve the problems of difficult industrial production, high cost of ligands, and small dosage, and achieve simple and easy-to-obtain raw materials and reagents, The effect of convenient purification and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
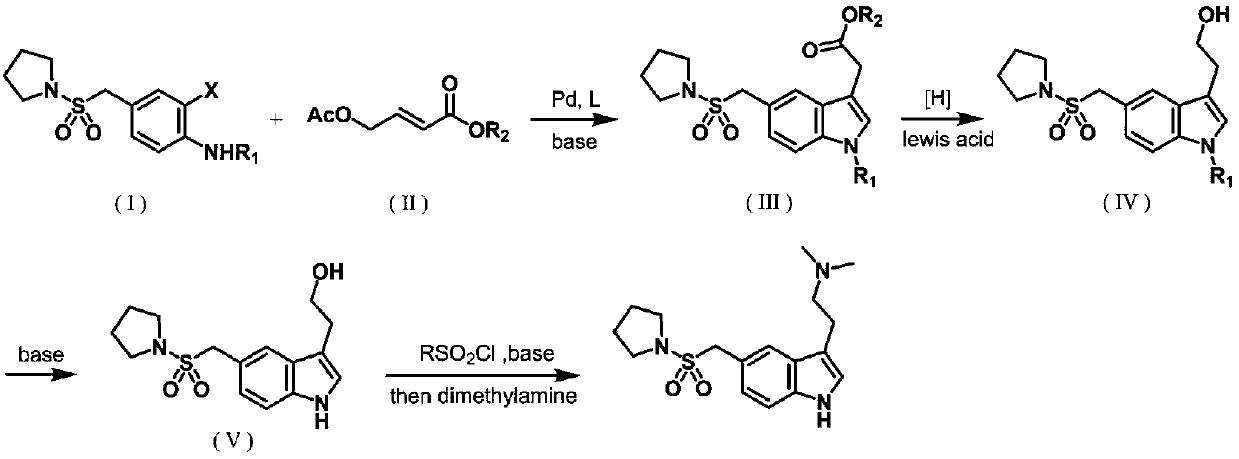
[0046] Preparation of ethyl 2-(5-((pyrrolidinyl-1-sulfonyl)methyl)-1-p-toluenesulfonyl-1H-indolyl-3-)acetate formula (Ⅲ)
[0047] Under nitrogen protection, N-(2-iodo-4-((pyrrolidinyl-1-sulfonyl) methyl) phenyl)-4-methylbenzenesulfonamide (formula (I) (26g, 50mmol ), 4-acetoxyethyl crotonate formula (Ⅱ) (8.6g, 50mmol), palladium acetate (0.23g, 1mmol), tri(o-methylphenyl)phosphine (0.6g, 2mmol), diisopropyl Add anhydrous N,N-dimethylacetamide (125ml) into a 250ml three-neck flask containing ethylethylamine (15.5g, 0.12mol) and a rotor, stir overnight in an oil bath at 100°C, and cool to room temperature after the reaction , add water (25ml), let stand in ice bath for 2h, filter, filter cake is washed with water (50ml) and ethanol (50ml) successively, and ethyl acetate is beaten to give white solid (formula (Ⅲ)) (19g, 38mmol). The rate is 76%.
[0048] h 1 NMR (400MHz, DMSO): 7.92(d,1H), 7.84(d,2H), 7.76(s,1H), 7.56(s,1H), 7.41-7.37(m,3H), 4.47(s,2H) ,4.09(q,2H),3.78(s,2H),...
Embodiment 2
[0050] Preparation of ethyl 2-(5-((pyrrolidinyl-1-sulfonyl)methyl)-1-p-toluenesulfonyl-1H-indolyl-3-)acetate formula (Ⅲ)
[0051] Under nitrogen protection, N-(2-bromo-4-((pyrrolidinyl-1-sulfonyl) methyl) phenyl)-4-methylbenzenesulfonamide (formula (I)) (2.4g , 5mmol), ethyl 4-acetoxycrotonate (formula (II)) (860mg, 5mmol), palladium acetate (45mg, 0.2mmol), tris(o-methylphenyl)phosphine (122mg, 0.4mmol), Add anhydrous N,N-dimethylacetamide (15ml) into a 50ml three-neck flask containing diisopropylethylamine (1.6g, 12mmol) and a rotor, stir overnight in an oil bath at 120°C, and cool after the reaction To room temperature, add water (3ml), let it stand in an ice bath for 2h, filter, wash the filter cake with water (6ml) and ethanol (6ml) successively, and beat with ethyl acetate to obtain a white solid (formula (Ⅲ)) (2.07g, 4.1 mmol), the yield is 80%.
Embodiment 3
[0053] 2-(5-((pyrrolidinyl-1-sulfonyl) methyl)-1-p-toluenesulfonyl-1H-indolyl-3-) preparation of ethyl acetate formula (Ⅲ) under nitrogen protection, to Equipped with N-(2-iodo-4-((pyrrolidinyl-1-sulfonyl)methyl)phenyl)-4-methylbenzenesulfonamide (formula (I)) (2.6g, 5mmol), 4 -Acetoxyethyl crotonate (formula (II)) (860mg, 5mmol), tris(dibenzylideneacetone)dipalladium) (183mg, 0.2mmol), tris(o-methylphenyl)phosphine (122mg, 0.4mmol), diisopropylethylamine (1.6g, 12mmol) and the 50ml three-neck flask of the rotor were added anhydrous N,N-dimethylacetamide (15ml), stirred overnight in an oil bath at 100°C, After the reaction, cool to room temperature, add water (3ml), let stand in an ice bath for 2h, filter, wash the filter cake with water (6ml) and ethanol (6ml) successively, and beat with ethyl acetate to obtain a white solid (formula (Ⅲ)) ( 1.96g, 3.9mmol), the yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com