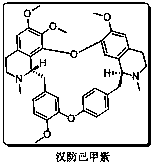
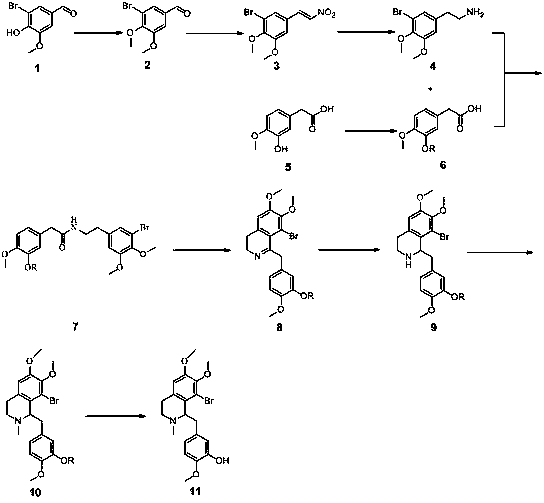
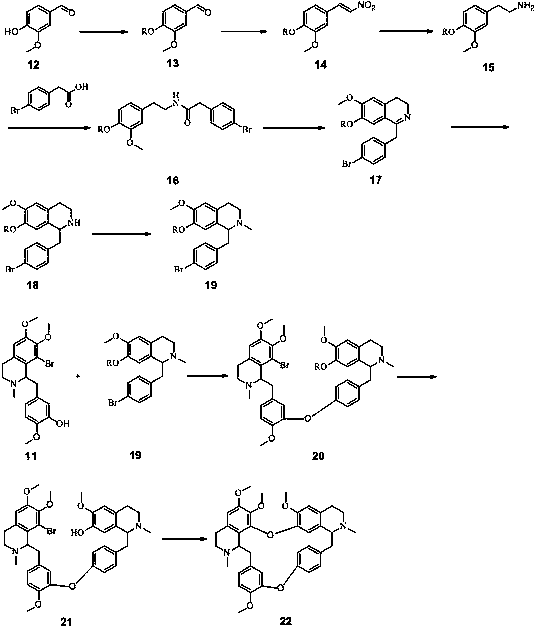
Full-synthetic method of racemic tetrandrine
A technology for total synthesis of tetrandrine, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of short supply, high pressure on environmental protection, low production efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) Preparation of Compound 2
[0075] Add 100 g of 5-bromovanillin and 1000 g of dichloromethane into a 3 L three-necked flask, stir at room temperature to dissolve and clarify. Add 200 g of 20% NaOH solution, and react at room temperature for 1 hour. Then add 100 g of methyl iodide, and heat to reflux for 10 hours, and the reaction of the raw materials is complete. The reaction solution was lowered to room temperature, left standing for liquid separation, and the aqueous phase was extracted twice with 200 g of dichloromethane. The dichloromethane phases were combined, washed once with 500 g of saturated brine and 500 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain 90.3 g of a yellow solid, namely Compound 2, with a yield of 85.1%.
[0076] (2) Preparation of Compound 3
[0077] Add 100 g of compound 2, 62 g of triethylamine, 125 g of nitromethane and 500 g of absolute ...
Embodiment 2
[0113] (1) Preparation of Compound 2
[0114]Add 115 g of 5-bromovanillin and 1150 g of chloroform into a 3 L three-necked flask, stir at room temperature to dissolve and clarify. Add 200 g of 20% NaOH solution, and react at room temperature for 1 hour. Add 130 g iodomethane again, heat to reflux reaction for 10 hours, the reaction of raw materials is complete. The reaction solution was lowered to room temperature, left standing for liquid separation, and the aqueous phase was extracted twice with 200 g of chloroform. The chloroform phases were combined, washed once with 500 g of saturated brine and 500 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain 95.2 g of a yellow solid, namely Compound 2, with a yield of 78.0%.
[0115] (2) Preparation of compound 3
[0116] 131 g of compound 2, 62 g of potassium carbonate, 98 g of nitromethane and 500 g of acetic acid were added into a 2...
Embodiment 3
[0152] (1) Preparation of Compound 2
[0153] Add 50 g of 5-bromovanillin and 500 g of toluene into a 1 L three-necked flask, stir at room temperature to dissolve and clarify. Add 59 g of potassium carbonate and cool down to 0~5 oC. Slowly add 41 g of dimethyl sulfate, after the addition is complete, heat to 40 °C for 14 hours, and the reaction of the raw materials is complete. The reaction solution was concentrated to dryness under reduced pressure, 200 g of water was added, and extracted twice with 500 g of dichloromethane. The dichloromethane phases were combined, washed once with 200 g of saturated brine and 200 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain 43.1 g of a yellow solid, namely Compound 2, with a yield of 81.2%.
[0154] (2) Preparation of Compound 3
[0155] 24.5 g of compound 2, 8.2 g of ammonium acetate, 20 g of nitromethane and 125 g of isopropanol were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com