Absorbable implantable device
An implantable and device technology, applied in the field of medical devices, can solve the problems of accelerating the metal matrix and the inability to effectively control the early degradation speed of the degradable polyester layer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
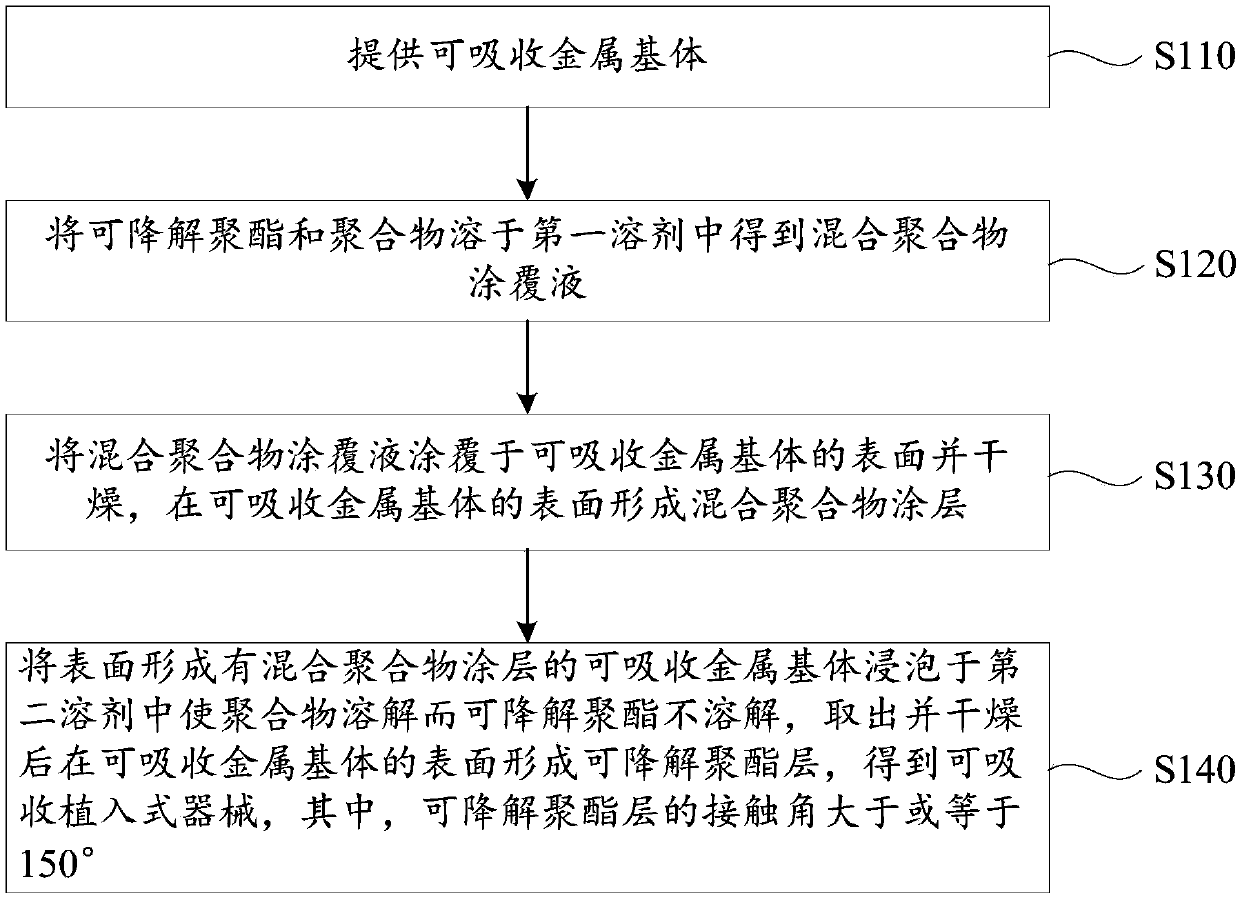
[0046] see figure 1 , a method for preparing an absorbable implantable device, comprising the steps of:
[0047] S110: Provide an absorbable metal matrix.
[0048] The shape and material of the absorbable metal matrix are the same as above, and will not be repeated here.
[0049] S120: Dissolving the degradable polyester and the polymer in the first solvent to obtain a mixed polymer coating liquid.
[0050] The polymer is selected from at least one of a degradable polymer and a non-degradable polymer, or is selected from at least one monomer forming the degradable polymer and at least one monomer forming the non-degradable polymer. At least one of the copolymers.
[0051] Preferably, the degradable polymer is selected from at least one of poly(racemic lactic acid), polypropylene carbonate, polycaprolactone, polyglycolic acid and polylactic acid-glycolic acid copolymer, and the non-degradable polymer is selected from polyphenylene At least one of ethylene, polymethyl methac...
Embodiment 1
[0087] Provide a pure iron luminal stent matrix with a thickness of 50 microns, mix and dissolve poly(racemic lactic acid) (PDLLA) and polylactic acid (PLLA) with a mass ratio of 2:3 in chloroform, and prepare a concentration of 4 mg / ml The mixed polymer coating liquid of PDLLA and 6mg / ml PLA is coated with the mixed polymer coating liquid on the outer surface of the pure iron luminal stent base by ultrasonic atomization spraying method, and after vacuum drying, it is applied on the pure iron tube A mixed polymer coating is formed on the outer surface of the luminal stent matrix, and then the pure iron luminal stent matrix formed with a mixed polymer coating on the outer surface is soaked in pharmaceutical grade ethyl acetate and treated at room temperature for 15 minutes to make the PDLLA in the polymer coating After dissolving, taking out and vacuum drying, a degradable polyester layer is formed on the surface of the pure iron luminal stent matrix to obtain the absorbable vas...
Embodiment 2
[0091] Provide an iron-based alloy luminal stent matrix with a thickness of 55 microns, mix and dissolve polylactic acid (PLA) and polypropylene carbonate (PPC) with a mass ratio of 4:2 in tetrahydrofuran, and prepare the concentrations to be 2mg / ml The mixed polymer coating solution of PLA and 4mg / ml PPC is coated on the outer surface of the pure iron luminal stent base by ultrasonic atomization spraying method, and after vacuum drying, it is applied on the pure iron tube A mixed polymer coating is formed on the outer surface of the luminal stent matrix, and then the pure iron luminal stent with the mixed polymer coating formed on the outer surface is soaked in dimethyl sulfoxide and treated at 40°C for 10 minutes to dissolve the PPC, and then taken out. After natural drying, a degradable polyester layer was formed on the surface of the pure iron luminal stent base to obtain the absorbable vascular stent of this embodiment. The ultrasonic atomization spraying equipment is Med...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com