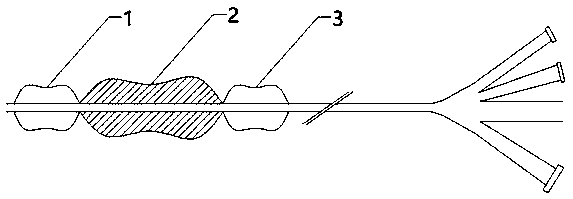
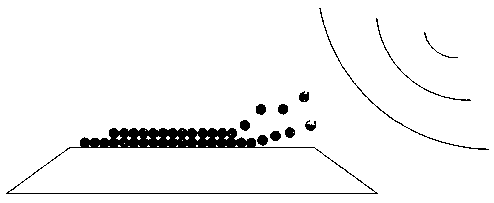
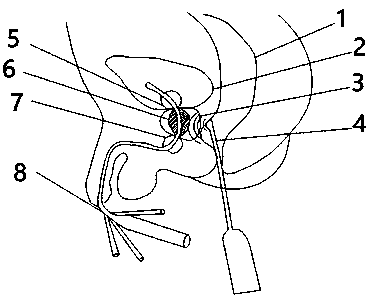
Ultrasonic controlled release prostate dilatation drug-delivery balloon system and preparation method thereof
A balloon and drug-loading technology, applied in the field of medical devices, can solve problems such as inability to load drugs and drain urine, unsatisfactory use and treatment effects, single function of balloon catheters, etc., to achieve good urethral dilatation and inhibition of benign prostatic hyperplasia, Improve the efficiency and effect, and the effect of treating benign prostatic hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0022] 1. Surface modification of ordinary balloons
[0023] 1. Use a 10.0*20 model (10.0mm in diameter and 20mm in length) to design serrated protrusions in the ordinary balloon forming mold. The sawtooth width is 0.5mm and the length is 18mm to make the mold required for non-compliant drug-loaded expansion balloon forming. .
[0024] 2. Use nylon raw materials to make a non-planar balloon according to conventional procedures and weld it on the balloon catheter.
[0025] 3. Perform plasma immersion treatment on the balloon segment of the balloon catheter to promote the adhesion of the drug coating. The specific steps are: place the catheter in the plasma immersion ion implantation device, after vacuuming, pass nitrogen gas, the gas pressure is 0.05~50 Pa; the radio frequency discharge power is 200~1200W; the amplitude of the loaded pulse high voltage power supply is 1KV~100KV, and the frequency is 50 ~40000 Hz or AC power 10-20 MHz, duty cycle 3%~80%; heating at 50~200℃ for...
specific Embodiment 2
[0039] 1. Surface modification of ordinary balloons
[0040] 4. Use a 10.0*20 model (diameter 10.0mm and length 20mm) to design serrated protrusions in the ordinary balloon forming mold. The sawtooth width is 0.5mm and the length is 18mm to make the mold required for non-compliant drug-loaded expansion balloon forming. .
[0041] 5. Use nylon raw materials to make a non-planar balloon according to conventional procedures and weld it on the balloon catheter.
[0042] 6. Perform plasma immersion treatment on the balloon segment of the balloon catheter to promote the adhesion of the drug coating. The specific steps are: place the catheter in the plasma immersion ion implantation device, after vacuuming, pass nitrogen gas, the gas pressure is 0.05~50 Pa; the radio frequency discharge power is 200~1200W; the amplitude of the loaded pulse high voltage power supply is 1KV~100KV, and the frequency is 50 ~40000 Hz or AC power 10-20 MHz, duty cycle 3%~80%; heating at 50~200℃ for 0.1~5...
specific Embodiment 3
[0056] 1. Surface modification of ordinary balloons
[0057] 7. Use a 10.0*20 model (10.0mm in diameter and 20mm in length) to design serrated protrusions in the ordinary balloon forming mold. The sawtooth width is 0.5mm and the length is 18mm to make the mold required for non-compliant drug-loaded dilation balloon forming. .
[0058] 8. Use nylon raw materials to make a non-planar balloon according to conventional procedures and weld it on the balloon catheter.
[0059] 9. Perform plasma immersion treatment on the balloon segment of the balloon catheter to promote the adhesion of the drug coating. The specific steps are: place the catheter in the plasma immersion ion implantation device, after vacuuming, pass nitrogen gas, the gas pressure is 0.05~50 Pa; the radio frequency discharge power is 200~1200W; the amplitude of the loaded pulse high voltage power supply is 1KV~100KV, and the frequency is 50 ~40000 Hz or AC power 10-20 MHz, duty cycle 3%~80%; heating at 50~200℃ for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com