Single-component long-wavelength photoinitiators and preparation method thereof
A photoinitiator, one-component technology, applied in the direction of organic chemistry, can solve the problems of short wavelength, mercury pollution, etc., and achieve the effects of reduced dosage, high molar extinction coefficient, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Weigh 1 mol of ethyl carbazole formaldehyde and 1 mol of acetylfuran, dissolve them in 20 ml of ethanol, and mix them evenly under stirring.
[0037] 2) Add 3-5 drops of ammonia solution with a mass fraction of 5% to the mixed solution prepared in step 1), adjust the pH value to 13, react at 30° C. for 3 hours, feed nitrogen gas, and continue the reaction for 3 hours in an ice bath. Pale yellow crystals precipitated.
[0038] 3) The light yellow crystal in step 2) is washed with ethanol, and the solvent is removed by vacuum drying to obtain a pure light yellow product, which is N-ethylcarbazoleethylene-furanone.
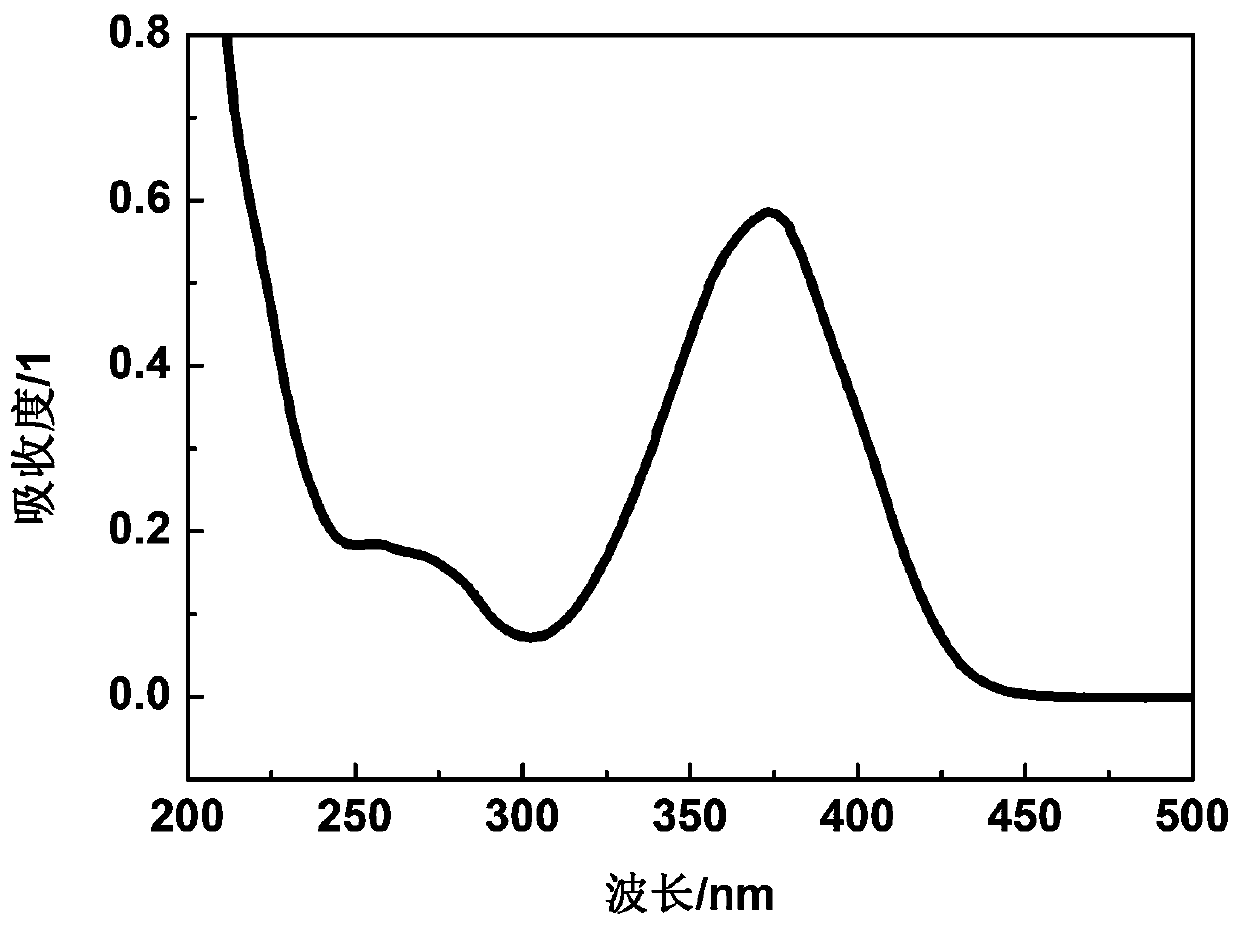
[0039] Dissolve N-ethylcarbazoleethylene-furanone in chromatography-grade acetonitrile at a concentration of 1 × 10 -5 mol / L, test its UV absorption, get its UV absorption spectrum as figure 2 As shown, the maximum absorption wavelength can reach 425nm.
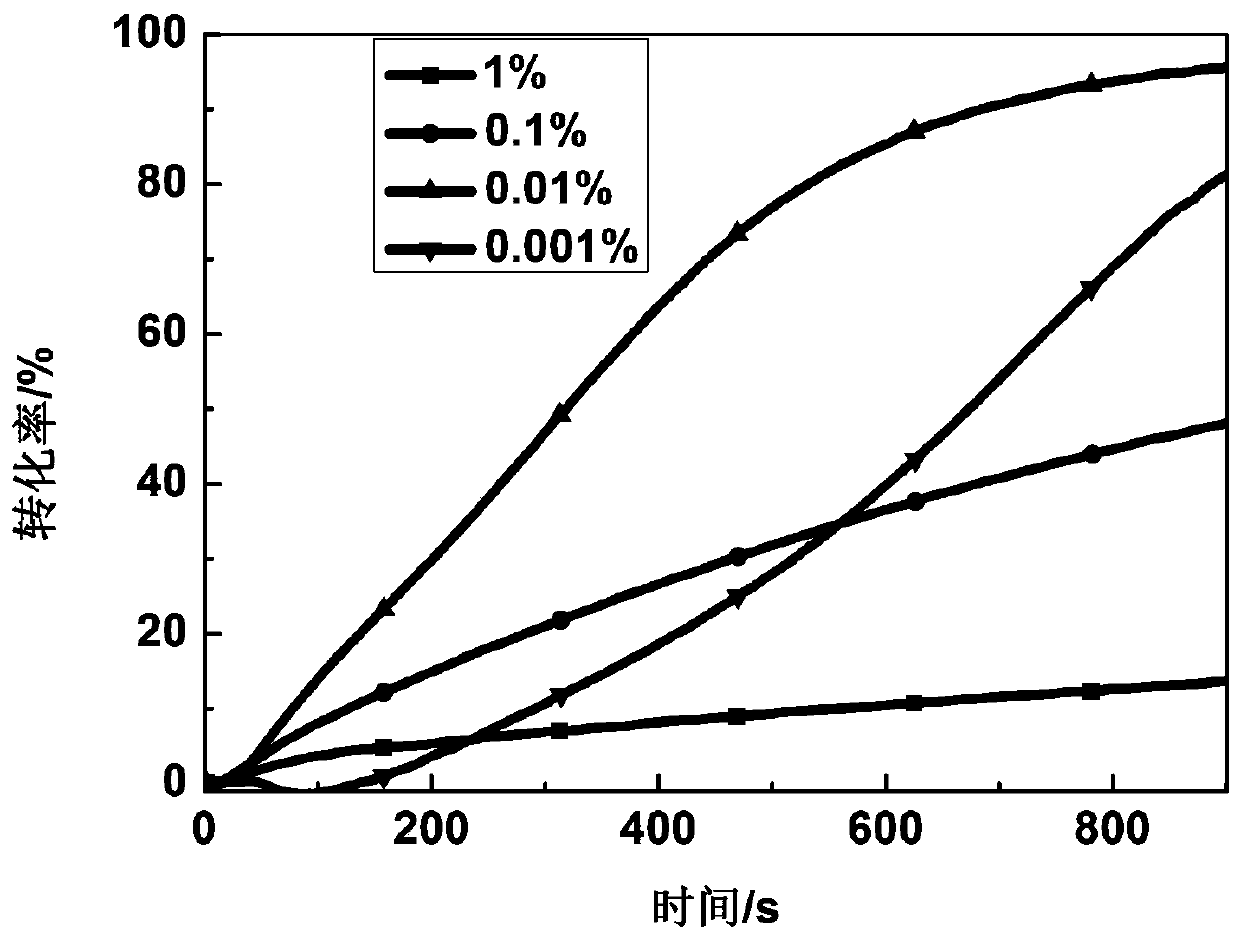
[0040] Add N-ethylcarbazoleethylene-furalone to PEGDA and HDDA at a mass concentration of 1%, 0.1%,...
Embodiment 2
[0042] 1) Weigh 1mol of ethylcarbazole formaldehyde and 1mol of acetylbenzene and dissolve in 20ml of ethanol, and mix evenly under stirring.
[0043] 2) Add 3-5 drops of ammonia solution with a mass fraction of 5% to the mixed solution prepared in step 1), adjust the pH to 13, react at 30°C for 3 hours, feed nitrogen gas, continue the reaction for 3 hours in an ice bath, and precipitate Pale yellow crystals.
[0044] 3) Washing the light yellow crystal in step 2) with ethanol, drying in vacuo to remove the solvent to obtain a pure light yellow product, the synthesized product is N-ethylcarbazoleethylene-benzophenone.
[0045] Dissolve N-ethylcarbazoleethylene-benzophenone in chromatography-grade acetonitrile at a concentration of 1 x 10 -5 mol / L, test its UV absorption. The results show that its ultraviolet absorption wavelength range is 250nm-420nm.
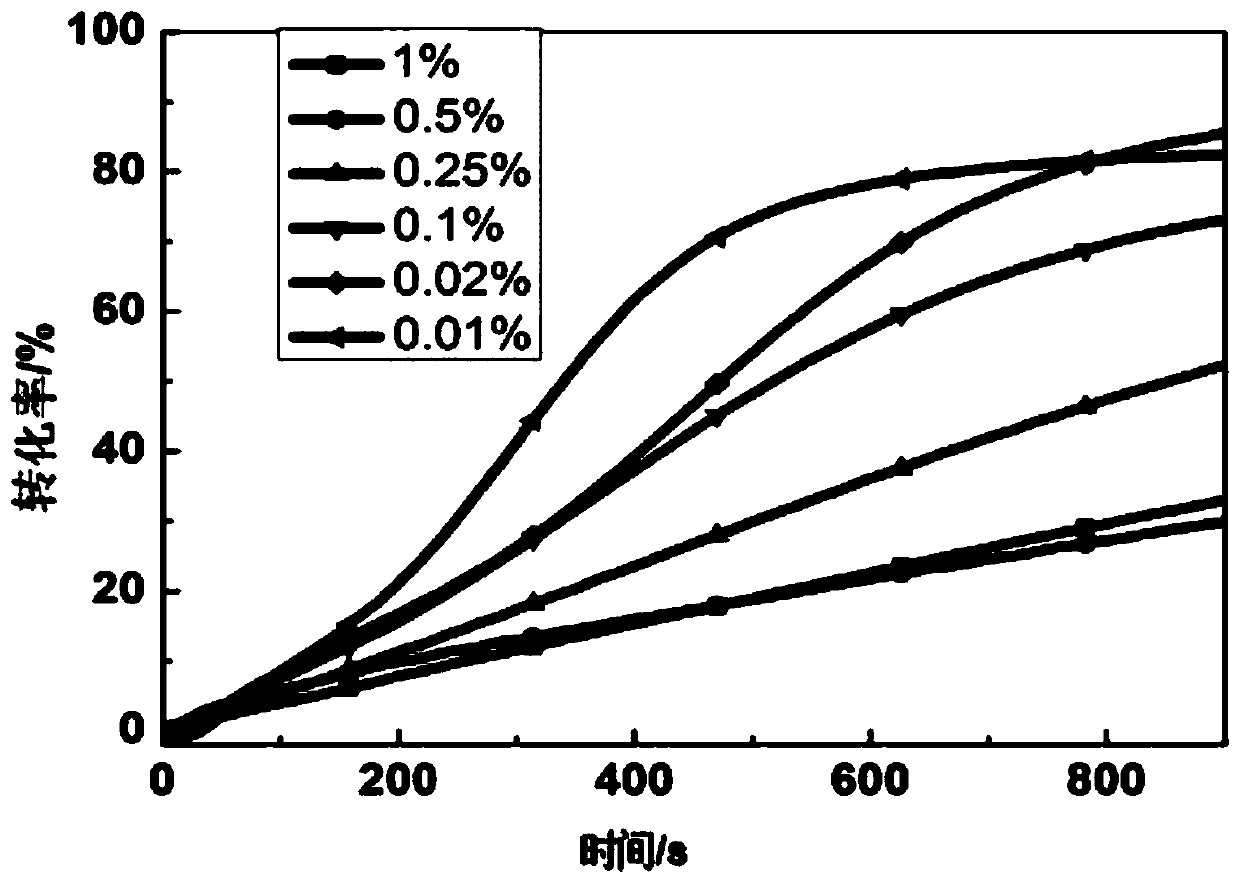
[0046] Add N-ethylcarbazoleethylene-benzophenone to PEGDA and HDDA monomers at a mass concentration of 1%, 0.1%, 0.01%, an...
Embodiment 3
[0048] 1) Weigh 1 mol of ethylcarbazole formaldehyde and 1 mol of acetylthiophene, dissolve them in 20 ml of ethanol, and mix them evenly under stirring.
[0049] 2) Add 3-5 drops of ammonia solution with a mass fraction of 5% to the mixed solution prepared in step 1), adjust the pH value to 13, react at 30° C. for 12 hours, feed nitrogen gas, and continue the reaction for 3 hours in an ice bath. Pale yellow crystals precipitated.
[0050] 3) Washing the light yellow crystal in step 2) with ethanol, drying in vacuo to remove the solvent to obtain a pure light yellow product, the synthesized product is N-ethylcarbazoleethylene-thienyl ketone.
[0051] Dissolve N-ethylcarbazoleethylene-thiophenemethanone in chromatography-grade acetonitrile at a concentration of 1 × 10 -5 mol / L, test its UV absorption. Its ultraviolet absorption wavelength range is 250nm-420nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com