Fuel cell separator
A fuel cell and separator technology, which is applied in the direction of fuel cells, fuel cell parts, power system fuel cells, etc., can solve the problem of increased contact resistance between the separator and the power generation unit, easy oxidation, titanium substrate and conductive carbon film Resisting problems such as weak adhesion, and achieving the effect of suppressing the increase in contact resistance and improving corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
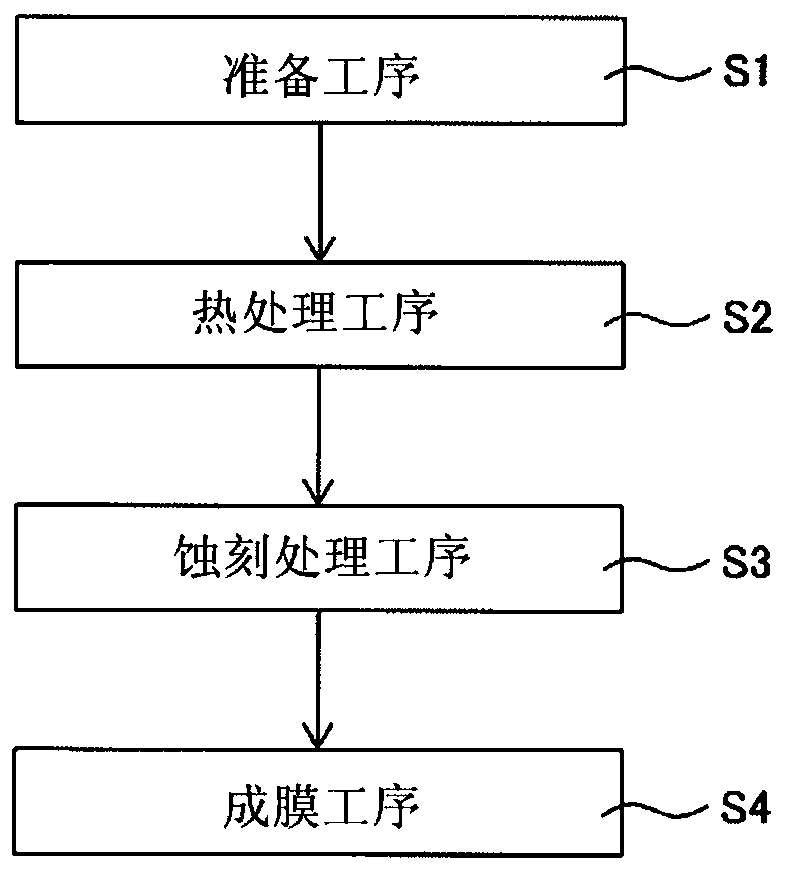
[0033] Hereinafter, the structure of this invention is demonstrated in detail based on an example of embodiment shown in drawing. Hereinafter, as an example, a case where the present invention is applied to a fuel cell mounted on a fuel cell vehicle or a fuel cell system including the same will be described, but the scope of application is not limited to such an example.
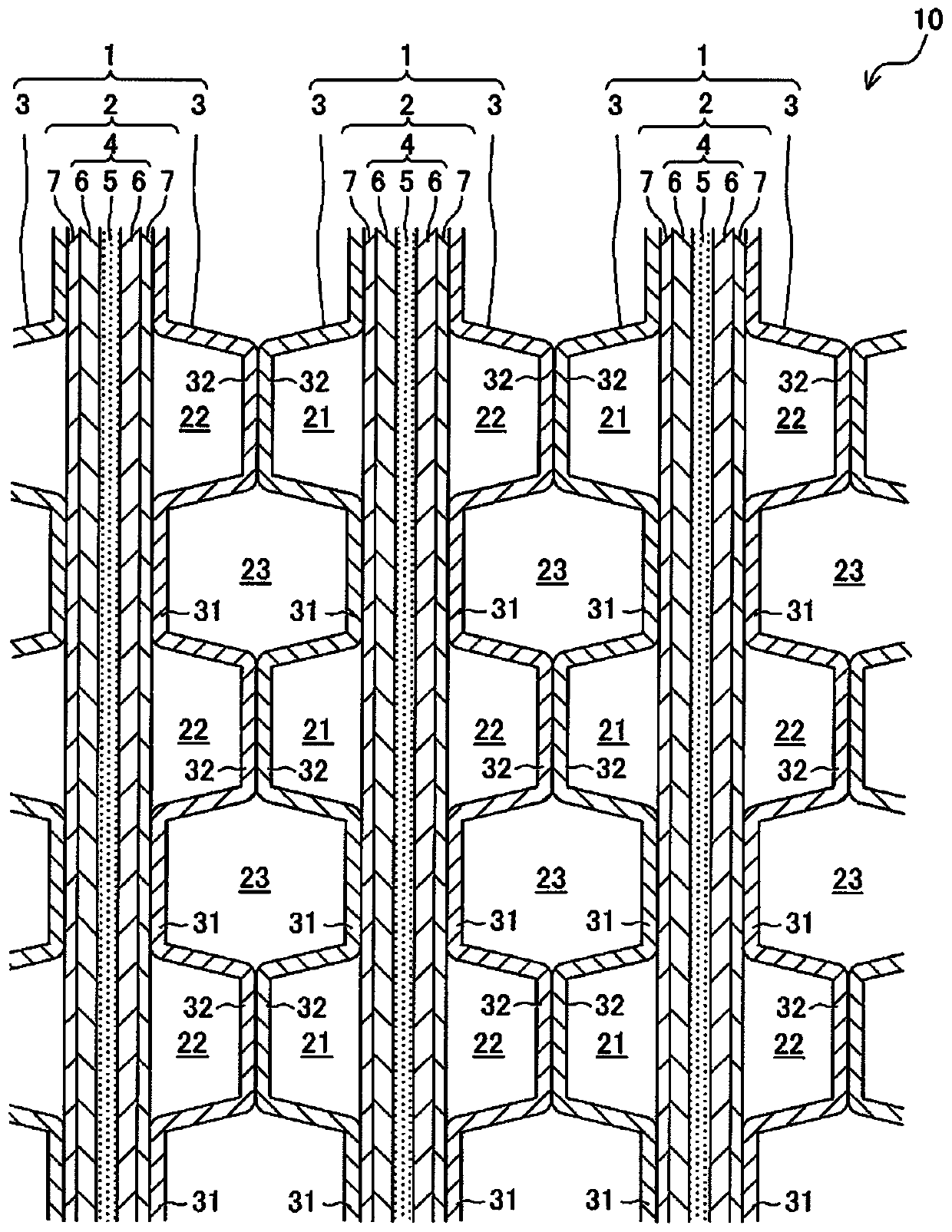
[0034] 1. Fuel cell 10 including separator 3
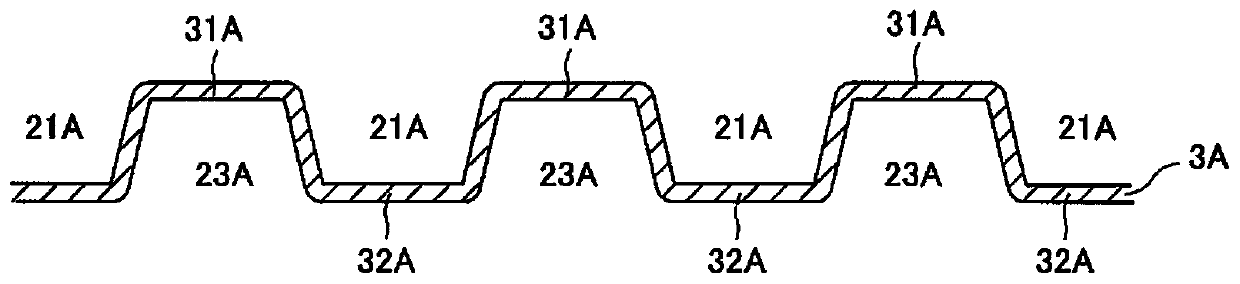
[0035] figure 1 It is a schematic cross-sectional view of a main part of a fuel cell 10 including a separator 3 according to an embodiment of the present invention. like figure 1 As shown, a fuel cell (fuel cell stack) 10 is stacked with a plurality of cells (single cells) 1 as basic units. Each cell 1 is a solid polymer fuel cell that generates an electromotive force through an electrochemical reaction between an oxidant gas (for example, air) and a fuel gas (for example, hydrogen gas). The battery 1 includes: a MEGA (Membrane Electrode & Gas Diffusion Laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com