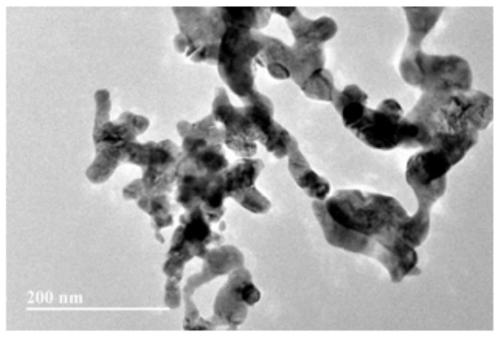
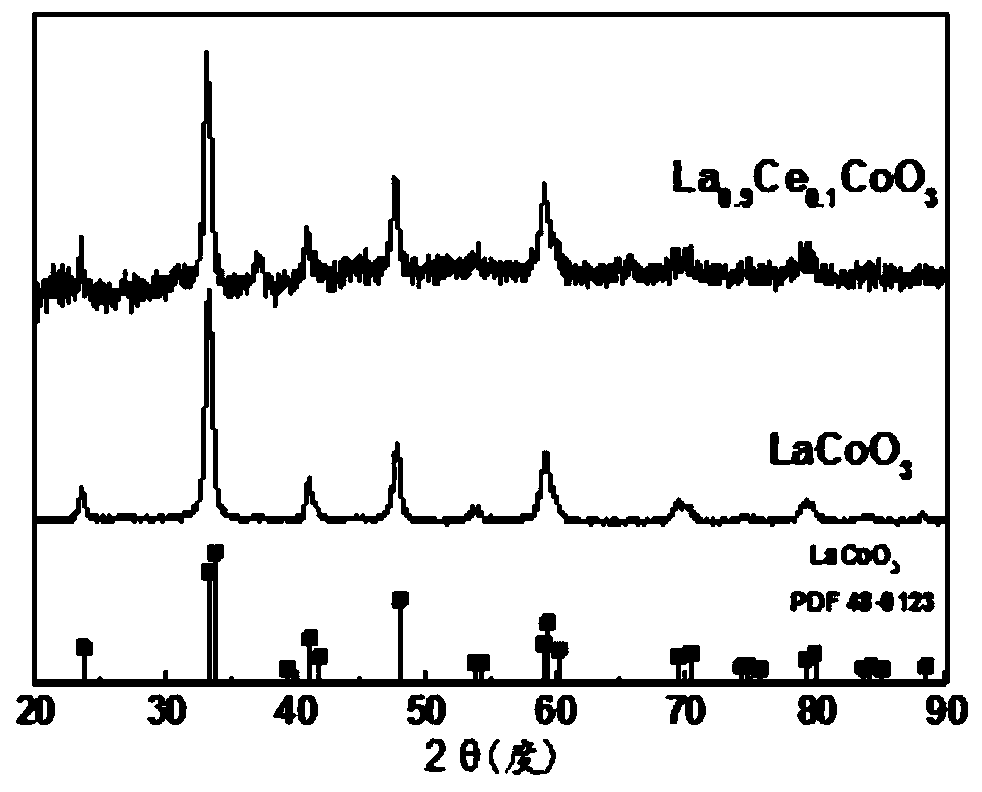
Network doped perovskite catalyst and preparation method and application thereof
A doped, perovskite technology, applied in chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve problems such as difficult to meet oxygen electrodes, unfavorable oxygen transmission, etc., to promote Improvement, good application prospects, and the effect of promoting catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment provides a network A-site doped perovskite catalyst La by chemical deposition. 0.9 Ce 0.1 CoO 3 , prepared according to the following steps:
[0044] (1) Take lanthanum nitrate, cerium nitrate and cobalt nitrate according to the stoichiometric ratio and dissolve them in deionized water until a uniform solution is formed.
[0045] (2) by adding a concentration of 1mol L -1 The pH of the homogeneous solution was adjusted to 9 with lithium hydroxide alkaline solution, and the stirring was continued for 2 hours at a stirring speed of 300 rpm. Centrifuge at a speed of 10000rpm for 10min to obtain a solid product, use a mixed solution of ethanol and deionized water to centrifuge and wash the solid product for 3 times, and dry at a temperature of 60°C for 12h to obtain a composite hydroxide precursor .
[0046] (3) The composite hydroxide precursor prepared in step (2) is placed in a tube furnace and roasted at 600°C for 2 hours in an air atmosphere to obt...
Embodiment 2
[0049] This embodiment 2 provides a network B-site doped perovskite catalyst LaCo 0.2 Fe 0.8 o 3 , prepared according to the following steps:
[0050] (1) Take lanthanum nitrate, cerium nitrate and cobalt nitrate according to the stoichiometric ratio, dissolve them in deionized water, and perform ultrasonic treatment for 30 minutes to form a homogeneous solution.
[0051] (2) Adding concentration is 0.1mol L -1 The pH of the homogeneous solution was adjusted to 9 with lithium hydroxide solution and stirring was continued for 2 hours. Centrifuge at a speed of 10000rpm for 10min to obtain a solid product, use a mixed solution of ethanol and deionized water to centrifugally wash the solid product for 3 times, and dry it for 8h at a temperature of 60°C to obtain a composite hydroxide precursor .
[0052] (3) The composite hydroxide precursor prepared in step (2) is placed in a tube furnace and calcined at 600°C for 2 hours in an air atmosphere to obtain a networked Co-doped L...
Embodiment 3
[0055] This embodiment 3 provides a network B-site doped perovskite catalyst LaCo 0.2 Fe 0.8 o 3 , prepared according to the following steps:
[0056] (1) Take lanthanum nitrate, iron nitrate and cobalt nitrate according to the stoichiometric ratio and dissolve them in deionized water until a uniform solution is formed.
[0057] (2) The pH of the homogeneous solution was adjusted to 9 by adding 10% aqueous ammonia alkaline solution, and the stirring was continued for 2 hours at a stirring speed of 300 rpm. Centrifuge at a speed of 10000rpm for 10min to obtain a solid product, use a mixed solution of ethanol and deionized water to centrifuge and wash the solid product for 3 times, and dry at a temperature of 60°C for 12h to obtain a composite hydroxide precursor .
[0058] (3) The composite hydroxide precursor prepared in step (2) is placed in a tube furnace and calcined at 600°C for 2 hours in an air atmosphere to obtain a network doped perovskite catalyst LaCo 0.2 Fe 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com