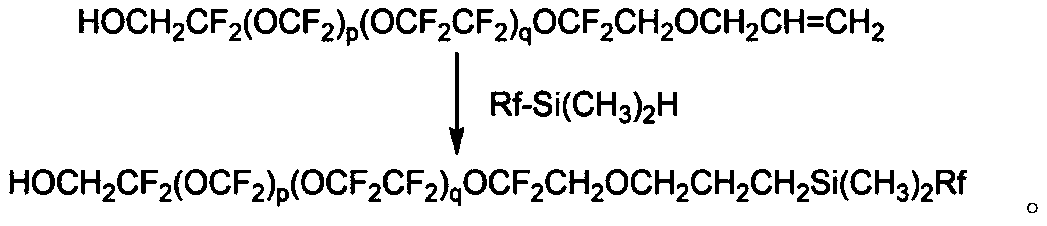
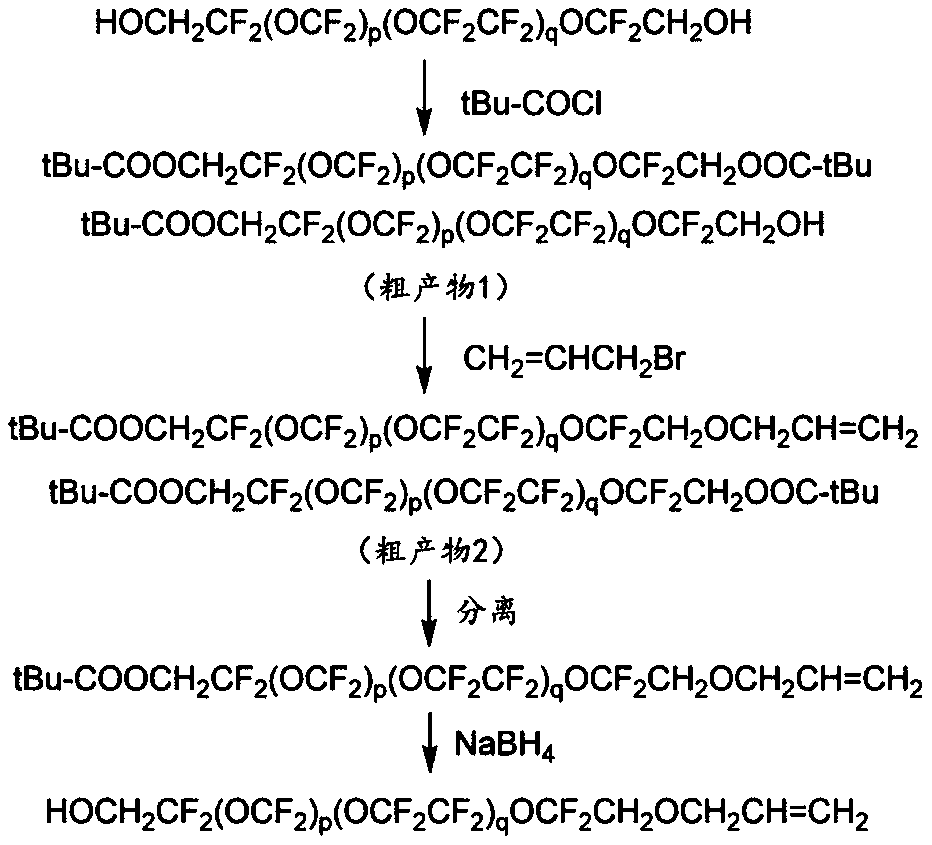Method for using perfluoroalkyl-containing chain-terminated double-terminal functional group perfluoropolyether for producing single-terminal functional group perfluoropolyether
A technology containing fluoroalkyl chain and perfluoropolyether, which is applied in polyether coatings, paints containing biocides, coatings, etc. The problems of poor lubricity of ether siloxanes can achieve the effects of excellent waterproof and oil-proof and anti-rubbing properties, excellent lubricity and anti-rubbing properties, and low coefficient of dynamic friction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) Preparation of α-hydroxy-ω-allyl-perfluoropolyether
[0070] Synthesis of HOCH 2 CF 2 (OCF 2 ) p (OCF 2 CF 2 ) q OCF 2 CH 2 OCH 2 CH=CH 2
[0071] At -5~5°C, add 10g (0.004mol) double-terminated hydroxyl perfluoropolyether (Mw=2500g / mol) and 0.61g (0.006mol) triethylamine into the three-necked flask, Stir in the cold bath circulating pump for 10-20 minutes. Then slowly add 0.53g (0.0044mol) of pivaloyl chloride dropwise to the mixture, and the dropwise addition is completed within 30min. The temperature was raised to room temperature, and stirring was continued at room temperature for 1 hour, then gradually warmed to 40°C, and stirred for 4 hours. After the reaction is completed, add water and fluorine solvent, stir rapidly for about 3-5 minutes, and a relatively clear organic phase appears. Finally, phase-separation extraction and vacuum distillation to remove the solvent gave 9.3 g of light yellow and clear crude product 1.
[0072] Weigh 7g (about ...
Embodiment 2
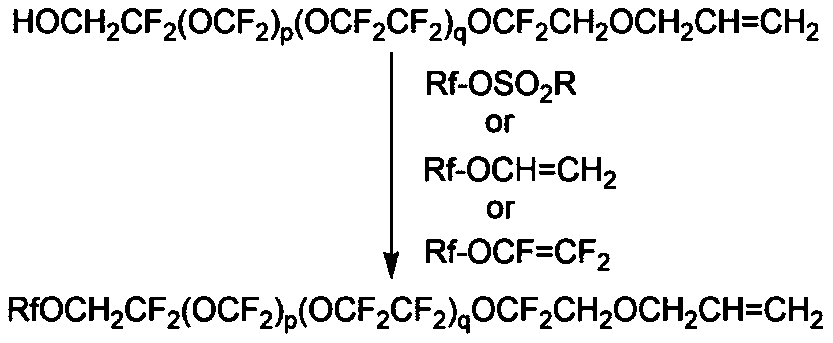
[0081] Synthetic C 3 f 7 OCHFCF 2 OCH 2 CF 2 (OCF 2 ) p (OCF 2 CF 2 ) q OCF 2 CH 2 OCH 2 CH=CH 2
[0082] 0.0.37g (0.0014mol) perfluoro-n-propyl vinyl ether and 3g (0.0012mol) α-hydroxyl-ω-allyl-perfluoropolyether in Example 1 were added to a closed stainless steel reactor, and then Add 0.36g of 20%wt (0.0018mol) sodium hydroxide aqueous solution, and stir the reaction at 60°C for 8h. After the reaction, water and fluorine solvent were added to the product, and stirred rapidly for 10 minutes, and a relatively clear organic phase appeared. Finally, phase-separation extraction and vacuum distillation yielded 2.5 g of light yellow clear single-ended perfluoropolyether allyl ether.
Embodiment 3
[0084] Synthetic C 3 f 7 O(C 3 f 6 O) 4 CF 2 CF 2 CH 2 O-
[0085] CH 2 CF 2 (OCF 2 ) p (OCF 2 CF 2 ) q OCF 2 CH 2 OCH 2 CH=CH 2
[0086] Add 3g (0.0012mol) of α-hydroxy-ω-allyl-perfluoropolyether and 0.035g (0.0014mol) of sodium hydride in Example 1 into a three-necked flask, and stir slowly at room temperature until the hydrogen is released Afterwards, the temperature was raised to 80° C., 3 ml of m-trifluorotoluene and 2 ml of N, N-dimethylformamide mixed solvent were added, and stirring was continued for 3 h. Then add 1.3g (0.0012mol) D-type perfluoropolyether p-toluenesulfonate, and stir and react at 120°C for 30h. After the reaction, water and fluorine solvent were added to the product, and stirred rapidly for 10 minutes, and a relatively clear organic phase appeared. Finally, phase-separation extraction and vacuum distillation yielded 3.6 g of brownish-yellow clear single-ended perfluoropolyether allyl ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com