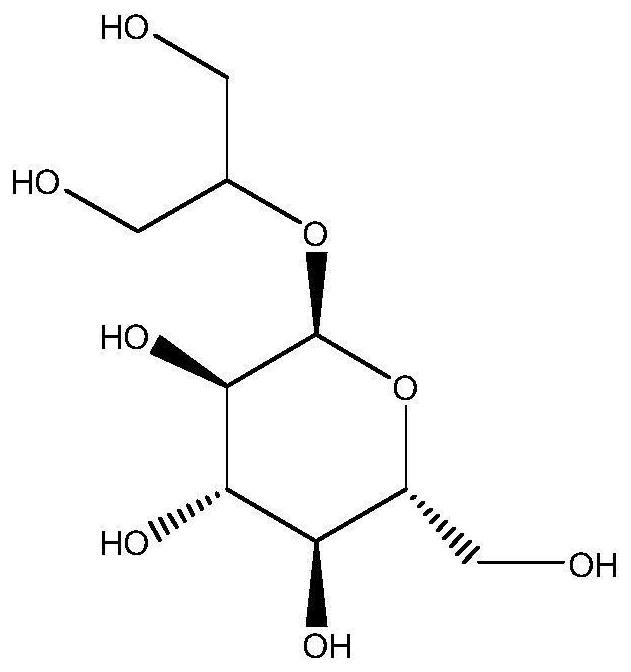
Application of a glycerol-2-α-glucosylase in the preparation of 2-α-glycerol glucoside
A technology of glucoside and glucose, applied in the application field of glycerol-2-α-glucosylase in the preparation of 2-α-glycerol glucoside, which can solve the limitations of 2-α-GG production technology, low yield, Difficult industrialization and other problems, to achieve the effect of separation and purification, high catalytic activity, high product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of the catalytic bacterial agent of producing 2-alpha-GG
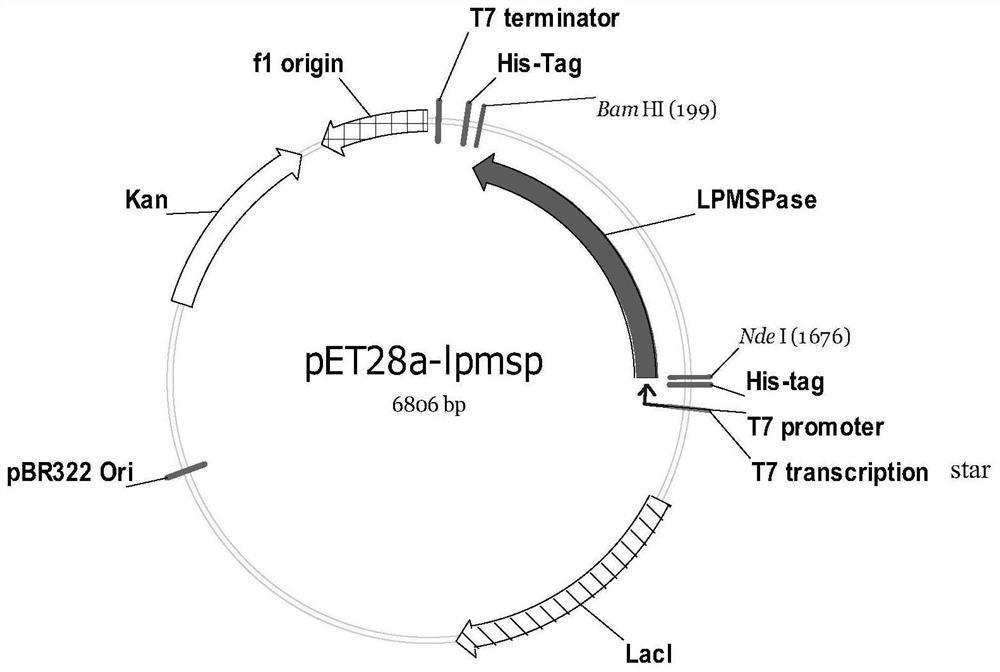
[0031] 1. Construction of Escherichia coli-lpmsp containing glycerol-2-α-glucosylase
[0032] Genomic DNA (NCBI accession number is MK370897) of Leuconostoc pseudosenteroides L. pseudomesenteroides in the mid-logarithmic growth phase was extracted using a bacterial genomic DNA extraction kit, and used as a template to perform PCR amplification with the following primers:
[0033] lpmSP-F:
[0034] GCCTGGTGCCGCGCGGCAGCCATATGGAAATTCAAAACAAAGCAATG;
[0035] lpmSP-R:
[0036] GTCGACGGAGCTCGAATTCGGATCCTTAGTTCTGAGTCAAATCATC.
[0037] The high-efficiency fidelity enzyme Phanta Max Super-Fidelity DNA Polymerase from Vazyme Biotech Co., Ltd. was used for PCR amplification. The PCR amplification program was: 95°C for 3min; 95°C for 15s, 58°C 15s at ℃, 1.5min at 72℃, 30 cycles; 5min at 72℃.
[0038] The obtained PCR product was purified using a PCR product recovery kit, and cloned between...
Embodiment 2
[0044] Application of embodiment 2 bacterial agents in the production of 2-alpha-GG
[0045] 1. Catalyst activity detection
[0046] Resuspend 1 g of the wet bacterial cells prepared by the method in Example 1 in 20 mL of pH 7.0, 2.5 mM phosphate buffer; add glycerol with a final concentration of 132 g / L and sucrose of 342 g / L, at 30 ° C and 220 rpm Catalyzed on a water bath shaker for 24 hours, the reaction solution was used for HPLC analysis, the measured residual substrate sucrose concentration was 18.81g / L, the formed product 2-α-GG concentration was 43.6g / L, and the substrate sucrose conversion rate was 94.5% .
[0047] Liquid chromatography detection conditions. Sample pretreatment: add 100 μL of reaction solution to 900 μL of 0.01mol / L dilute hydrochloric acid; centrifuge at 10000×g for 5 minutes, filter with a 0.22 μm filter membrane, and add the filtrate to a liquid phase vial; chromatographic column: Sugar-Ca column, 300×7.8mm; column temperature: 80°C, detector t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com