Method for determining concentration of loxoprofen and trans-hydroxyl matrix thereof in plasma
A technology of trans-hydroxyl body and concentration, applied in the field of biomedicine, can solve the problems of low sensitivity, long analysis time, difficult separation, etc., and achieve the effects of high sensitivity, good selectivity and shortened analysis time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the embodiments of the present invention. Apparently, the described embodiments are only some of the embodiments of the present invention, not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0016] The invention provides a technical scheme: a method for measuring the concentration of loxoprofen and its trans-hydroxy body in plasma, comprising the following steps:
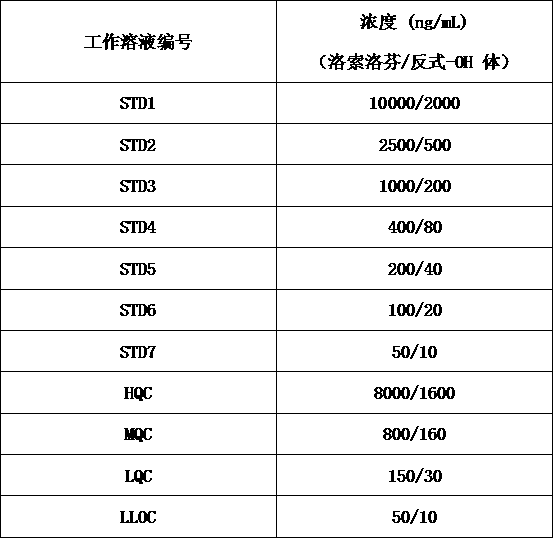
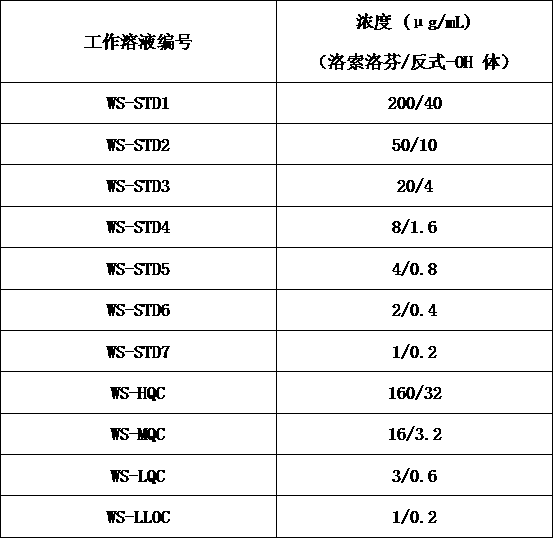
[0017] S1: Preparation of stock solution: use methanol:water (1:1, v:v) as diluent to dissolve loxoprofen and trans-OH loxoprofen reference substance to obtain loxoprofen with a concentration of 400 μg / mL Loxoprofen standard curve stock solution (STD Stock-LSLF) and loxoprofen quality control stock sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com