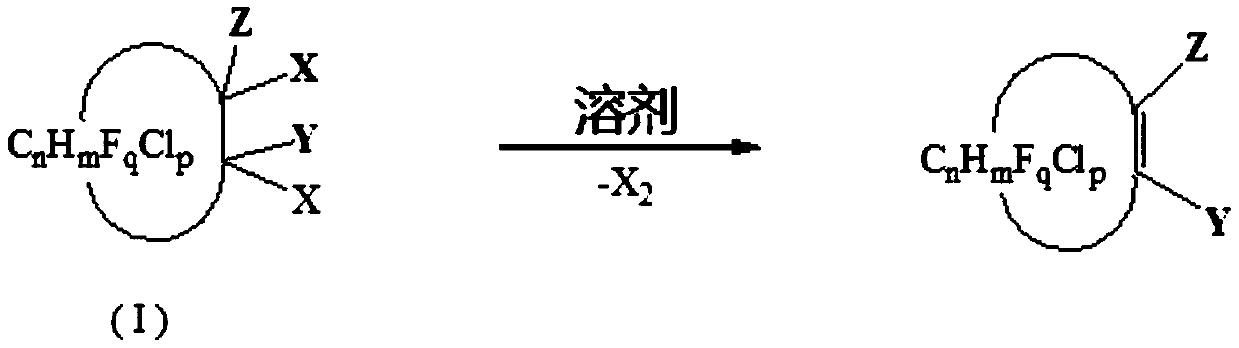
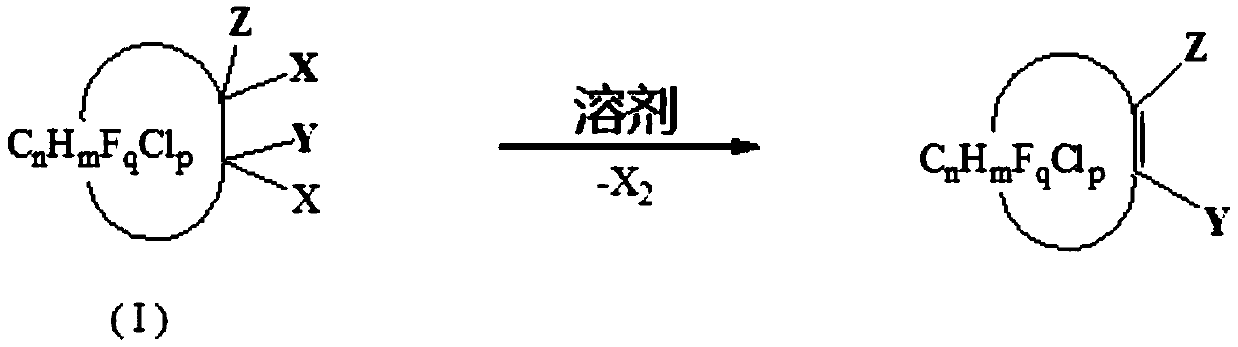
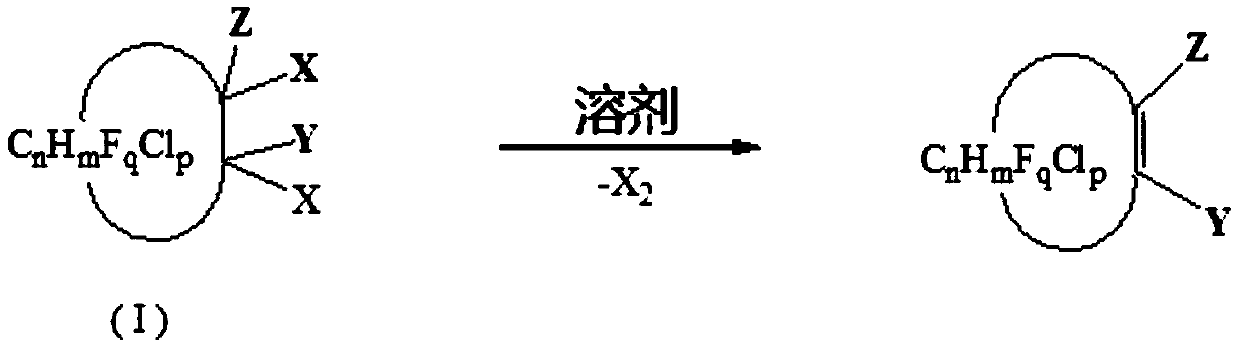
Preparation method of halogenation cycloolefin
A technology of halogenated cycloalkenes and halogenated cycloalkanes, which is applied in the field of preparation of halogenated cycloalkenes, can solve the problems of waste solids such as a large amount of metal halides, pollute the environment, and low yield, and achieve high yield and safe process Reliable results with mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In a 1L autoclave, add 2 mol of N,N-dimethylformamide and 1 mol of 1,2-dichlorohexafluorocyclobutane (the molar ratio of cis and trans is 60:40), at 160 The reaction was carried out for 6 hours. After the reaction was finished, rectification was carried out to obtain hexafluorocyclobutene (boiling point was 5-6°C / 760mmHg), the yield was 96.8%, and the purity was 99.1%.
Embodiment 2
[0057] In a 1L autoclave, add 2 mol of N,N-dimethylacetamide and 1 mol of 1,2-dichlorohexafluorocyclobutane (the molar ratio of cis and trans is 60:40), at 170 The reaction was carried out for 6 hours. After the reaction was finished, rectification was carried out to obtain hexafluorocyclobutene (boiling point was 5-6°C / 760mmHg), the yield was 95.8%, and the purity was 99.2%.
Embodiment 3
[0059] In a 1L autoclave, add 1 mol of N,N-dimethylformamide and 1 mol of 1,1,2-trichloro-2,3,3-trifluorocyclobutane, and react at 160 degrees for 6 hours, After the reaction, rectification was carried out to obtain 1-chloro-2,3,3-trifluorocyclobutene (boiling point: 51-52°C / 760mmHg), with a yield of 80.5% and a purity of 98.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com