Preparation method of nitrification organic matter and prepared nitrification organic matter
A technology of organic matter and nitrating acid solution, which is applied in the preparation of nitro compounds, organic chemistry, chemical instruments and methods, etc. It can solve the problems of affecting the quality of chemical products, poor batch production stability, and high post-processing costs, and achieve large-scale industrial applications. value, improved production environment, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
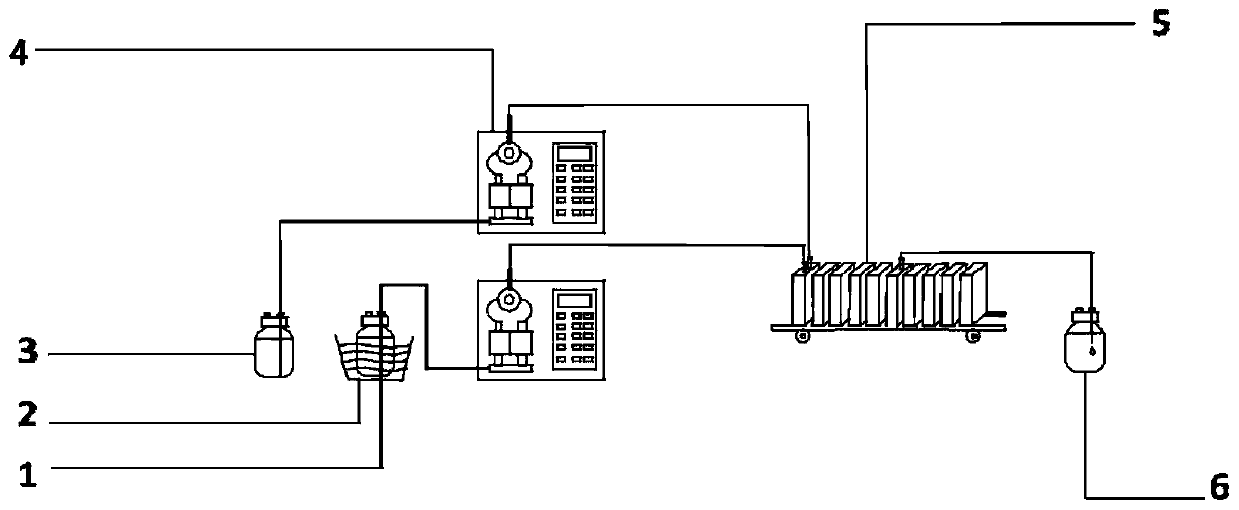
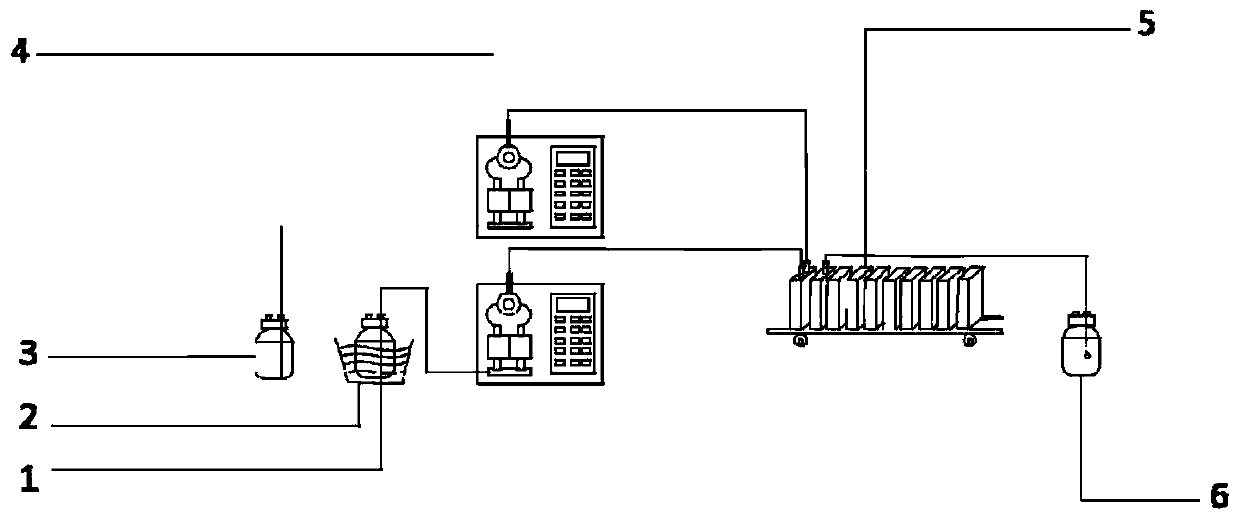
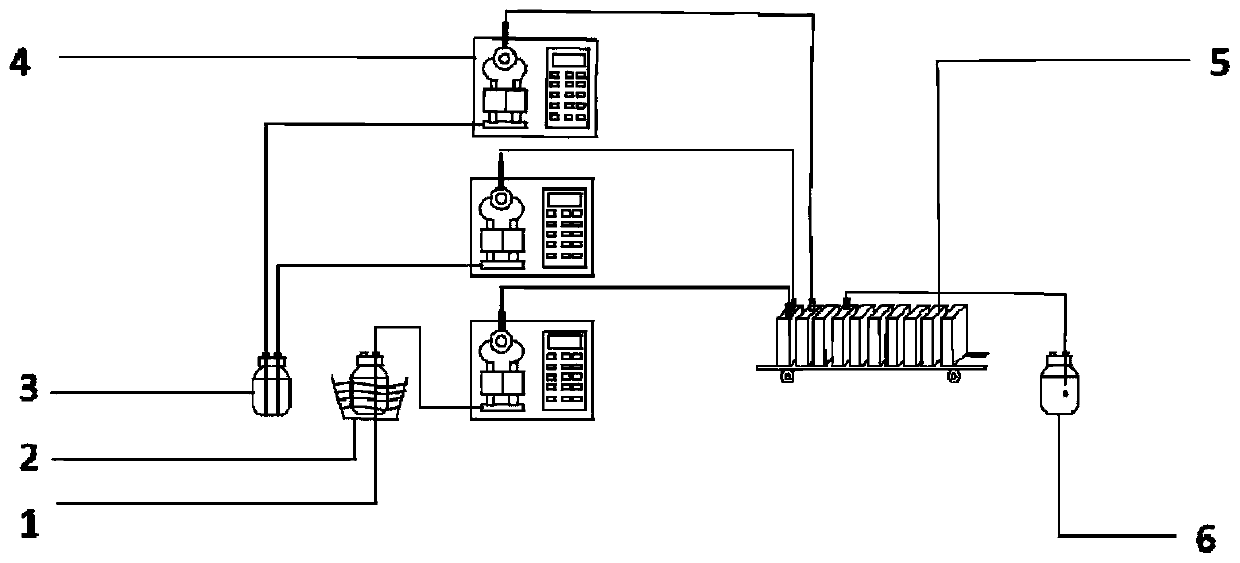
[0022] A kind of preparation method of nitrification organic matter provided by the embodiment of the present invention, it comprises the following steps: pass nitrification acid liquid and molten state to be nitrified organic matter into microchannel reactor to react; Preferably, reaction residence time is more than 10s, in order to Taking the cresol reaction as an example, the reaction principle is as follows:
[0023]
[0024] Wherein, the organic matter to be nitrated is solid at normal temperature, and after the organic matter to be nitrated in molten state is passed into the microchannel reactor, the organic matter to be nitrated will not solidify and / or dissolve in the nitrifying acid solution. In order to ensure the state of the organic matter to be nitrated in the microchannel reactor, the reaction temperature should not be too low. Preferably, the difference between the reaction temperature and the melting point temperature of the organic matter to be nitrated is X...
Embodiment 1
[0046] The present embodiment provides a kind of preparation method of nitrated organic matter (o-nitro-p-cresol), comprising the steps:
[0047] Connect two metal high-pressure constant-flow pumps to the first two feeding ports of the microchannel reactor, and wash them with solvent water. After the sampling bottle is cleaned and dried, it is connected to the outlet of the sixth piece of the microchannel reactor. After the microchannel reactor was rinsed with solvent, it was filled with 30% nitric acid solution.
[0048] The temperature of the microchannel reactor is set at 20°C, and the p-cresol is heated in a water bath or an incubator until it is molten, and the molar ratio of p-cresol and nitric acid is set to 1:1.3, and p-cresol and 30% nitric acid The flow rates of the solutions were 18.5mL / min and 41.5mL / min respectively, and they were mixed in the microchannel reactor, and the residence time was 60s (the effective liquid holding capacity was 60.0mL). During the reac...
Embodiment 2
[0050] The present embodiment provides a kind of preparation method of nitrated organic matter (o-nitro-p-cresol), comprising the steps:
[0051] Connect two metal high-pressure constant-flow pumps to the first two feeding ports of the microchannel reactor, and wash them with solvent water. After the sampling bottle is cleaned and dried, it is connected to the outlet of the sixth piece of the microchannel reactor. After the microchannel reactor was rinsed with solvent, it was filled with 30% nitric acid solution.
[0052] Set the temperature of the microchannel reactor to 30°C, place p-cresol in a water bath or incubator and heat it to 40°C to be in a molten state, set the molar ratio of p-cresol and nitric acid to 1:1.3, p-cresol and 30 The flow rate of % nitric acid solution is 18.5mL / min and 41.5mL / min respectively, enters the microchannel reactor and mixes, and the residence time is 60s (effective liquid holding capacity is 60.0mL). During the reaction process, the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com