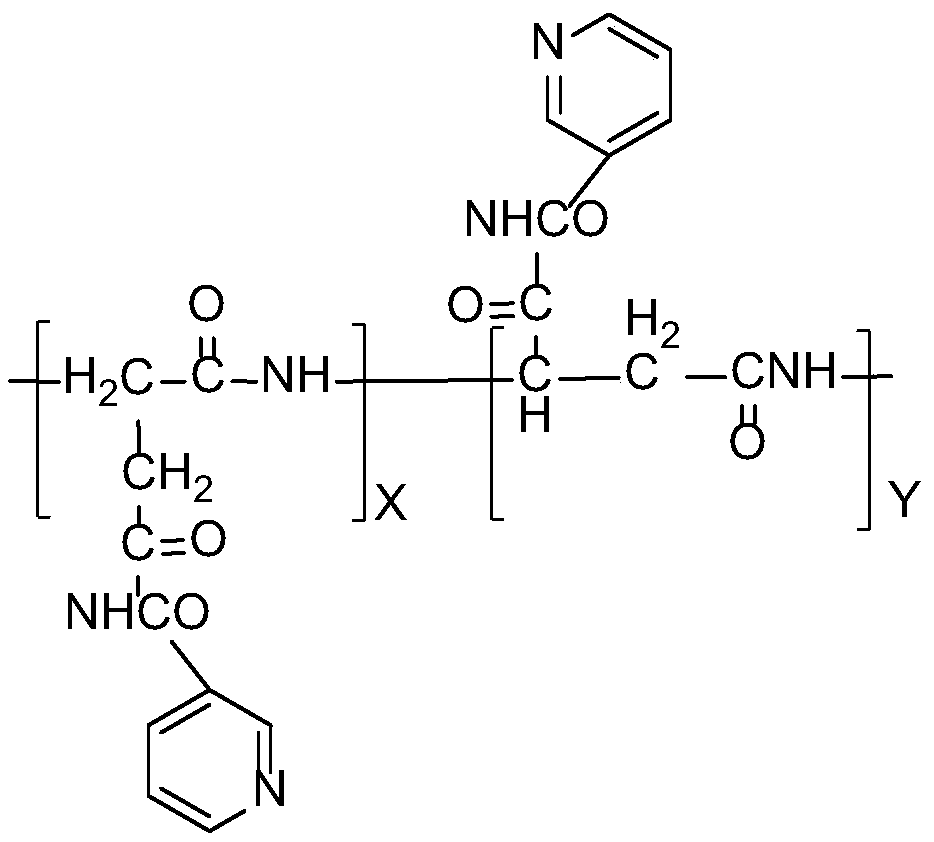
Synthesis method of nicotinamide modified polyaspartic acid derivative
A technology of polyaspartic acid and synthesis method, which is applied in the field of graft-modified polyaspartic acid derivative synthesis, can solve the problems of no polyaspartic acid derivative reports, etc., and achieve good hydrophilicity, The effect of improving skin texture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] A kind of synthetic method of the polyaspartic acid derivative of nicotinamide modification of the present invention, it comprises the following steps:
[0016] Step 1. Synthesis of polysuccinimide: add 100-150 parts of L-aspartic acid and 2-6 parts of phosphoric acid catalyst to the kneading reactor respectively, heat up and dehydrate and condense under vacuum conditions to obtain intermediate polysuccinimide Body (PSI);
[0017] Step 2, adding nicotinamide to the kneading reactor in step 1, maintaining the reaction conditions in step 1, and continuing the reaction to obtain a polysuccinimide derivative grafted with nicotinamide;
[0018] Step 3. Add the nicotinamide-modified polysuccinimide derivatives prepared in step 2 into the hydrolysis tank, add water 3-6 times the weight of the polysuccinimide derivatives, stir and disperse, and heat and maintain stirring Slowly add NaOH solution dropwise under the conditions for hydrolysis, and the hydrolyzed product is polyas...
Embodiment 1
[0025] Example 1: Add 100 parts of L-aspartic acid and 6 parts of phosphoric acid catalyst into the kneading reactor, dehydration condensation at 170°C and -70kPa for 2 hours to obtain polysuccinimide intermediate (PSI). Next, 20 parts of nicotinamide was added into the reactor, and the above reaction conditions were maintained to continue the reaction for 2 hours to obtain a nicotinamide-modified polysuccinimide derivative. Add the polysuccinimide derivative modified by nicotinamide into the hydrolysis tank, add water to stir and disperse, slowly add 3mol / L NaOH solution dropwise at 40°C and keep stirring for 2.5h to obtain nicotinamide graft modified polyaspartic acid saline solution. The aqueous solution is purified and further refined through steps such as pH adjustment, filtration, ultrafiltration, and concentration. The obtained nicotinamide-modified polyaspartic acid had a molecular weight of 15,000 and a grafting rate of 14%.
Embodiment 2
[0026] Example 2: Add 100 parts of L-aspartic acid and 3 parts of phosphoric acid catalyst into the kneading reactor, dehydration condensation at 185°C and -50kPa for 3 hours to obtain polysuccinimide intermediate (PSI). Next, 40 parts of nicotinamide was added into the reactor, and the above reaction conditions were maintained to continue the reaction for 1 hour to obtain a nicotinamide-modified polysuccinimide derivative. Add the polysuccinimide derivative modified by nicotinamide into the hydrolysis tank, add water to stir and disperse, slowly add 4 mol / L NaOH solution dropwise under the condition of maintaining stirring at 50°C for 5 hours, and obtain the nicotinamide graft-modified polysuccinimide derivative. Polyaspartic Acid Saline Solution. The aqueous solution is purified and further refined through steps such as pH adjustment, filtration, ultrafiltration, and concentration. The prepared nicotinamide-modified polyaspartic acid had a molecular weight of 18000 and a gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com