Liquid-phase synthesis method of semaglutide side chain
A liquid-phase synthesis and side-chain technology, which is applied in the field of liquid-phase synthesis of semaglutide side-chains, can solve the problem of not finding a pure liquid-phase synthesis method for the side-chain, and achieves easy control of the reaction process, simple production process, and easy process. workable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
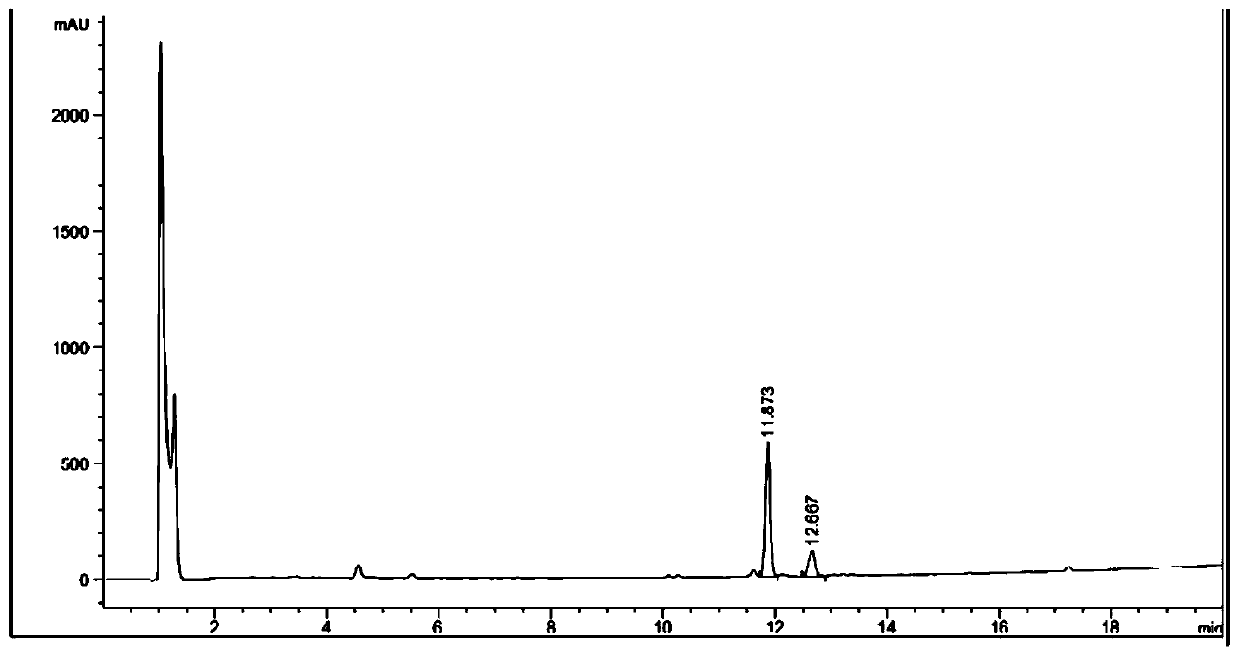
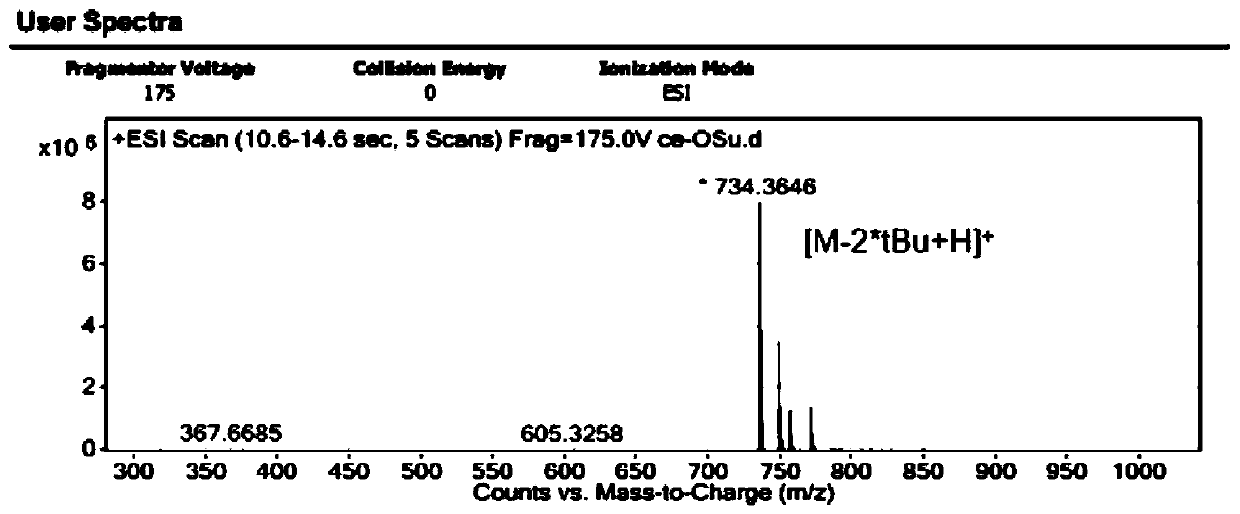
Image
Examples
Embodiment 1
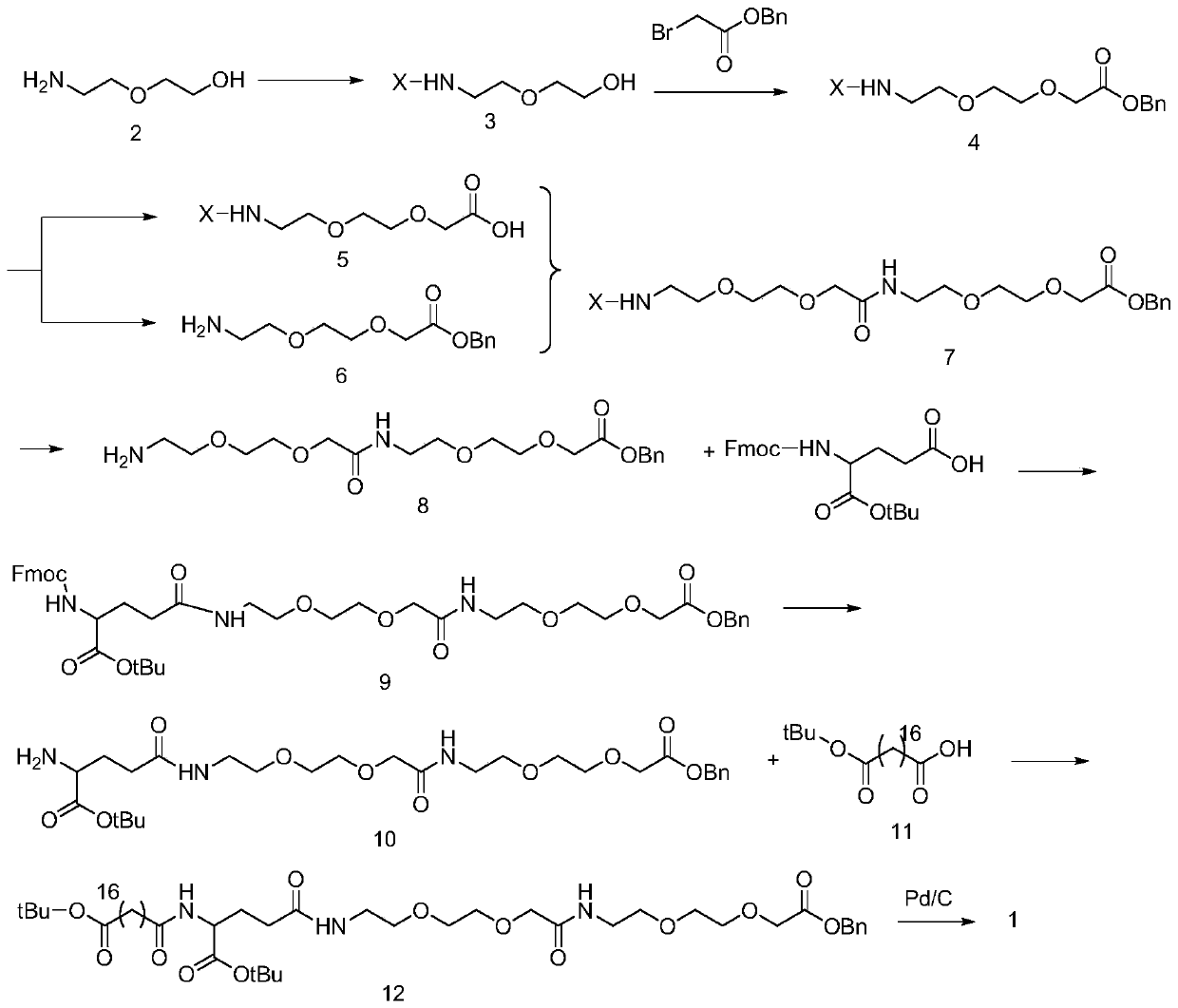
[0058] according to figure 1 The synthetic route shown carries out the liquid phase synthesis of the side chain of semaglutide:
[0059] a. Preparation process of compound 3
[0060] Dissolve 1.05g of compound 2 (10mmol) in 50ml of DCM solution, place it in a nitrogen-protected flask, and carefully add (Boc) 2 O (2.62 g, 12 mmol). The mixture was stirred for 12 hours, then the crude material was concentrated in vacuo. Purification by flash column chromatography gave a colorless oil in 95% yield.
[0061] b. The preparation process of compound 4:
[0062] Add 3.08g (9.5mmol) of compound 3, 8.6g (4eq) of benzyl bromoacetate and 2.75g (2.1eq) of potassium carbonate into a round-bottomed flask. Relatively complete; washed with water, extracted 2-3 times with EA, washed with saturated brine, anhydrous Na 2 SO 4 After drying, P:E=6:1, the product was collected and the yield was 85%.
[0063] c. The preparation process of compound 5:
[0064] Dissolve 1.412g (4mmol) of compo...
Embodiment 2
[0081] a. Preparation process of compound 3
[0082]Dissolve 5mmol / ml compound 2 (1ml) in DCM solution, place it in a nitrogen-protected flask, and after the above mixture drops to 0°C, add 1eq of Et dropwise 3 N; After 20 minutes, slowly add 2mmol / ml Trt-Cl (2.5ml) mixture to the above mixture, and remove the ice bath after 10 minutes; stir at room temperature, observe on the plate after 1h, the reaction is complete . Wash with water, extract with DCM 2-3 times, wash with saturated brine, anhydrous Na 2 SO 4 After drying, P:E=5:1, the product was collected and the yield was 85%.
[0083] b. The preparation process of compound 4:
[0084] Add 694mg (2mmol) of compound 3, 1832mg (4eq) of benzyl bromoacetate and 580mg (2.1eq) of potassium carbonate into a round-bottomed flask, and raise the reaction temperature to 50°C. After reacting for 18 hours, spot the plate and observe that the reaction is relatively complete; wash with water , EA extracted 2-3 times, washed with satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com