Berberine hydrochloride and ibuprofen eutectic substance as well as preparation method, composition and application thereof
A kind of technology of berberine hydrochloride and co-crystal, applied in the field of co-crystal formed by berberine hydrochloride and ibuprofen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation method 1 of berberine hydrochloride and ibuprofen cocrystal:
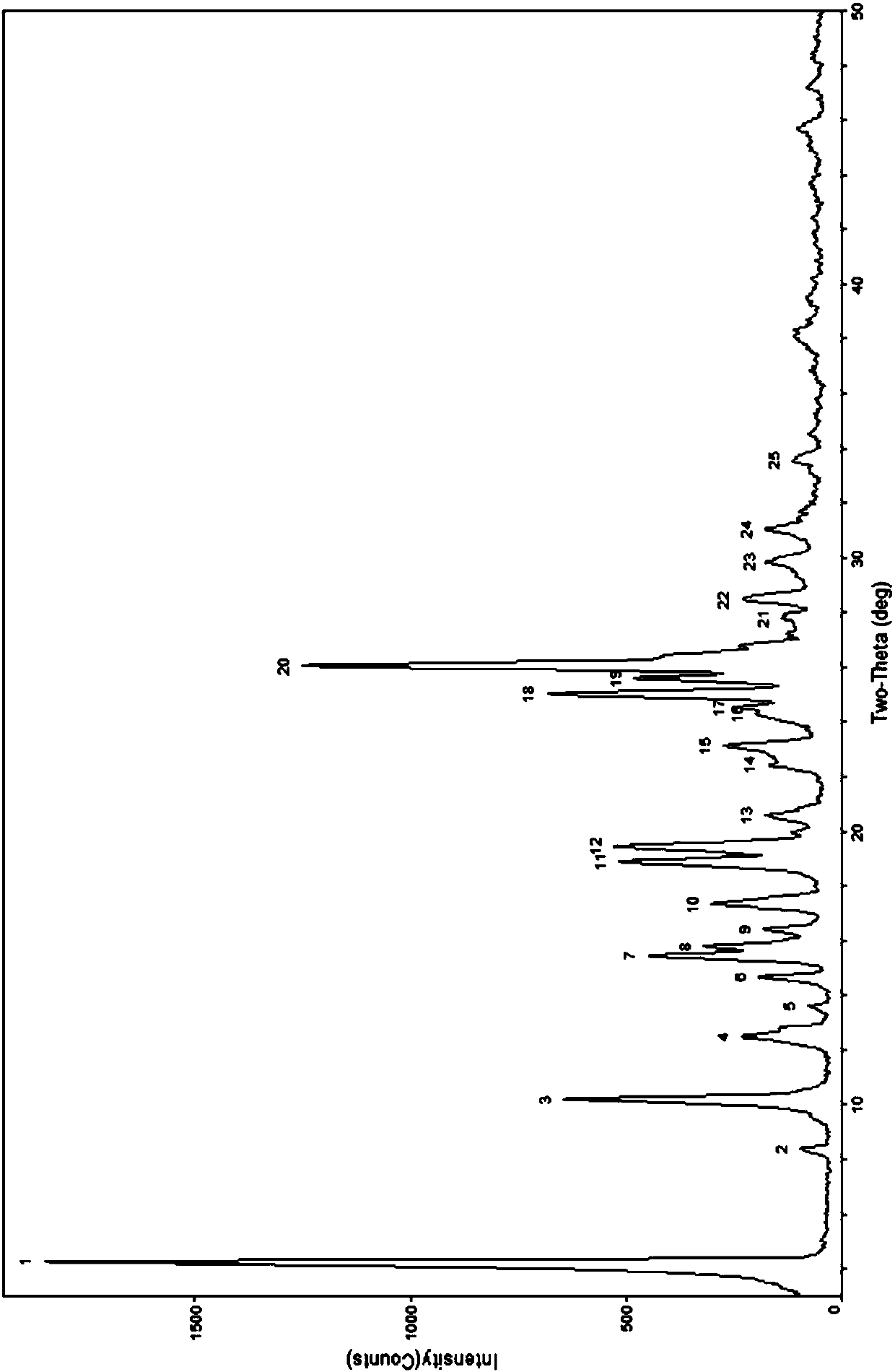
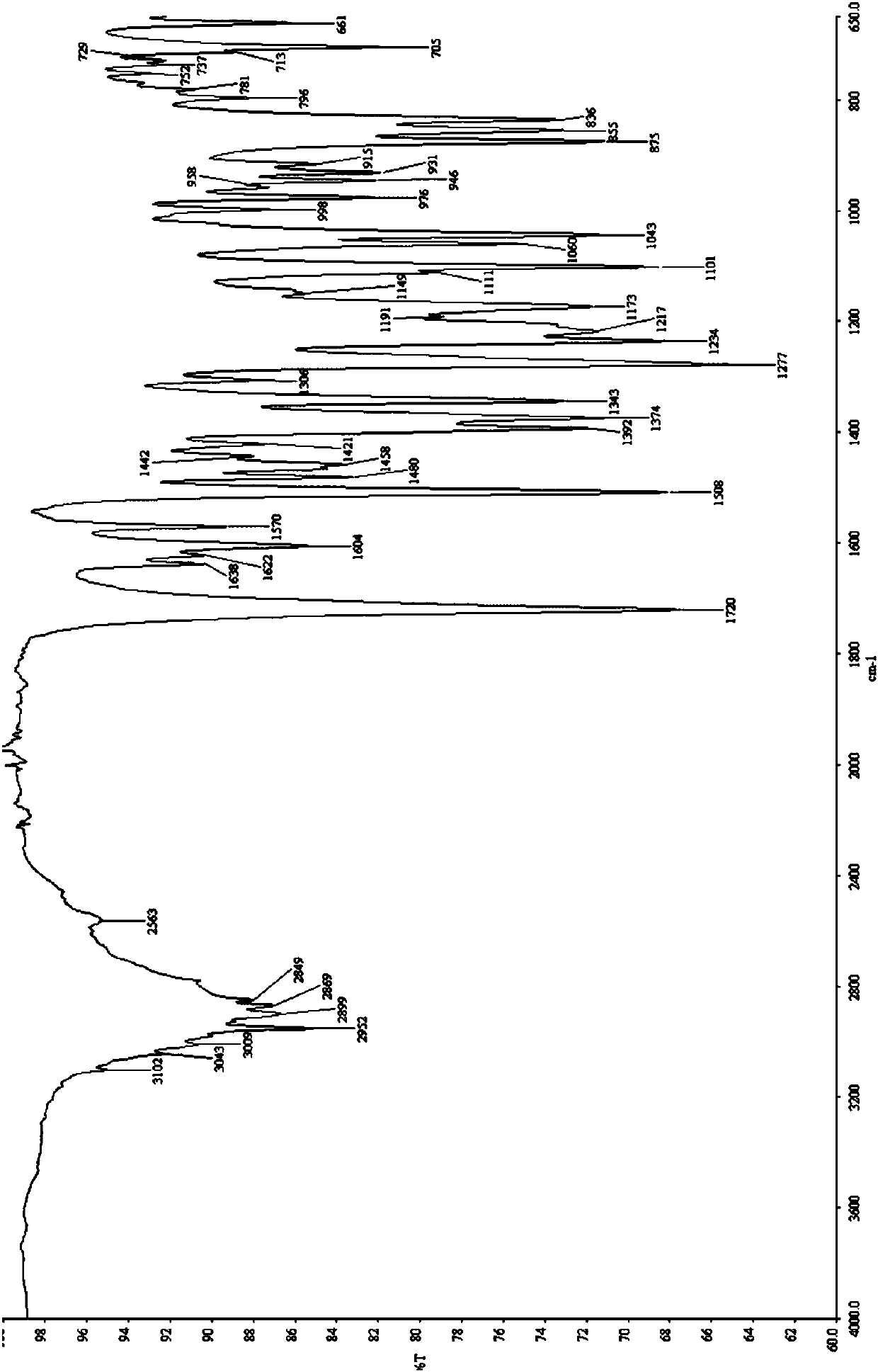
[0059] As shown in the table below, take an appropriate amount of berberine hydrochloride and ibuprofen and put them into a mortar with a molar ratio of 1:1, add an appropriate amount of organic solvent, and manually grind for an appropriate time. Carry out powder X-ray diffraction analysis to it, its diffraction pattern and figure 1 Consistent, indicating that the obtained sample is a cocrystal of berberine hydrochloride and ibuprofen.
[0060]
[0061] Preparation method 2 of berberine hydrochloride and ibuprofen cocrystal:
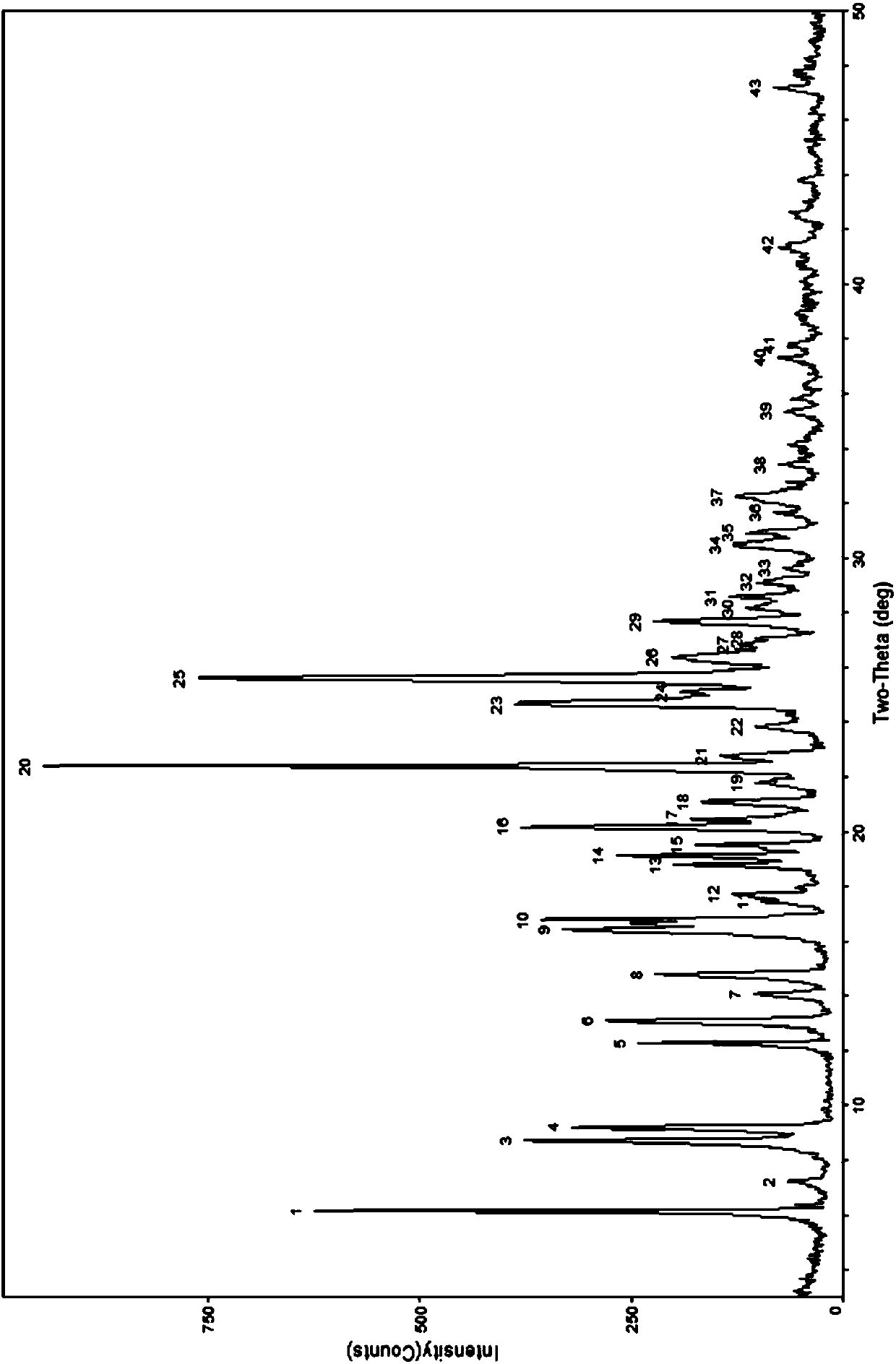
[0062] According to the table below, take appropriate amount of berberine hydrochloride and ibuprofen and put them into the ball mill tank according to the molar ratio of 1:1, add an appropriate amount of organic solvent, select an appropriate ball-to-material ratio, set an appropriate speed, and grind for an appropriate time. Carry out powder X-ray diffraction analys...
Embodiment 2
[0068] The solubility characteristics of berberine hydrochloride and ibuprofen co-crystal and berberine hydrochloride bulk drug in 0.1N hydrochloric acid solution and aqueous solution with pH value of 1.0 were investigated. Measured with reference to the "Technical Guidelines for Dissolution Test of Ordinary Oral Solid Preparations", the dissolution curve comparison adopts the model-independent similarity factor (f2) method, and the calculation of f2 value compares berberine hydrochloride and berberine hydrochloride with ibuprofen. The similarity of the dissolution curves of crystal samples in the two solvent systems, when the f2 value is higher than 50, the two curves are considered to be similar, and when the f2 value is lower than 50, the two curves are considered to be different. In the experiment, the berberine hydrochloride sample was used as a reference, and the model-independent similarity factor f2 was calculated. The dissolution percentage adopts the high performance...
Embodiment 3
[0073] Absorption characteristics and plasma concentration characteristics of berberine hydrochloride and ibuprofen co-crystal in rats:
[0074] Six SD rats were randomly divided into 2 groups, 3 rats in each group, and they were fasted without water for 12 hours before administration. Weigh the body weight of the rat, according to 100mg·kg -1 The berberine hydrochloride dosage calculation, the berberine hydrochloride and the berberine hydrochloride and ibuprofen co-crystal samples were loaded into the solid dispenser, and the drug powder was directly inserted into the stomach of the rat through the oral cavity. 5min, 15min, 30min, 45min, 1h, 1.5h, 2h, 4h, 8h, and 12h after administration, blood was collected from the inner canthus of the eye, placed in a heparinized tube, centrifuged at 4000rpm for 10min at 4°C, and frozen at -40°C In the refrigerator to be tested. Accurately measure 100 μL of plasma, put it into a 1.5ml EP tube, add 10 μL of internal standard carbamazepine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com