Polyacid Ag(I) coordination polymer as well as preparation method and application thereof
A technology of coordination polymers and acid groups, applied in the field of electrochemical catalysis, can solve the problems of undiscovered and less research on electrocatalytic activity, and achieve the effects of simple preparation process, good electrocatalytic activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Synthesis of polyacid-based Ag(I) coordination polymer
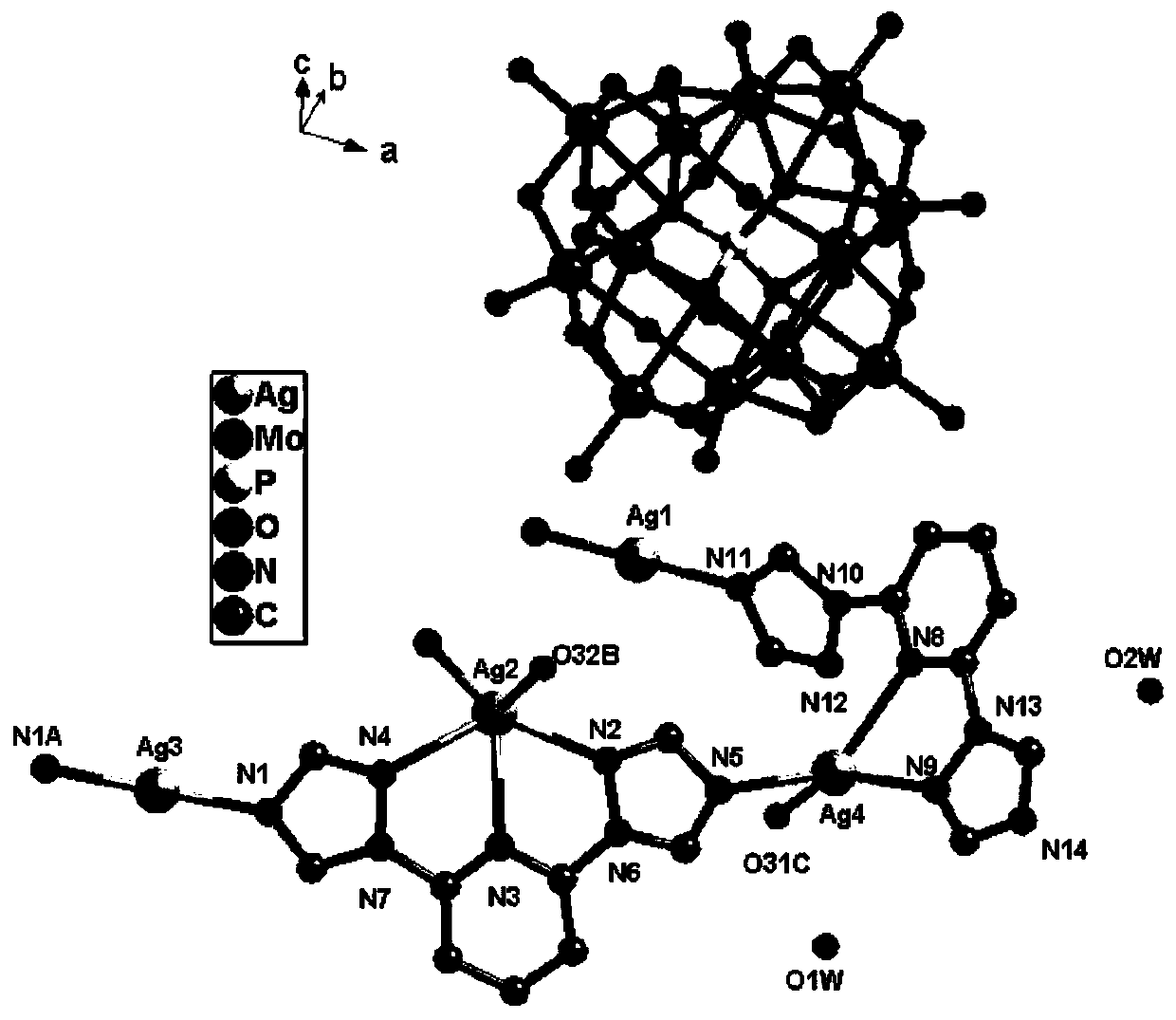
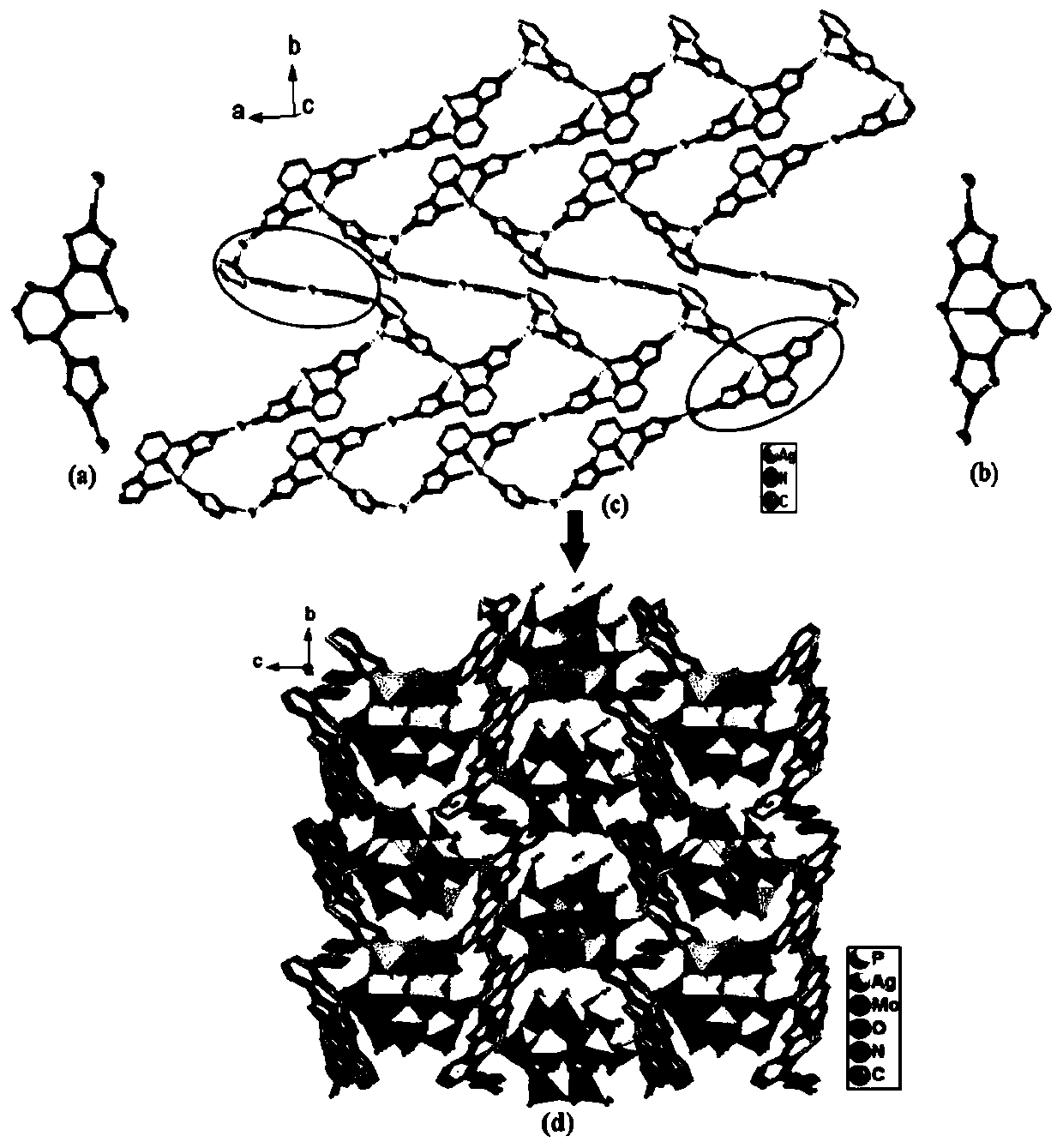
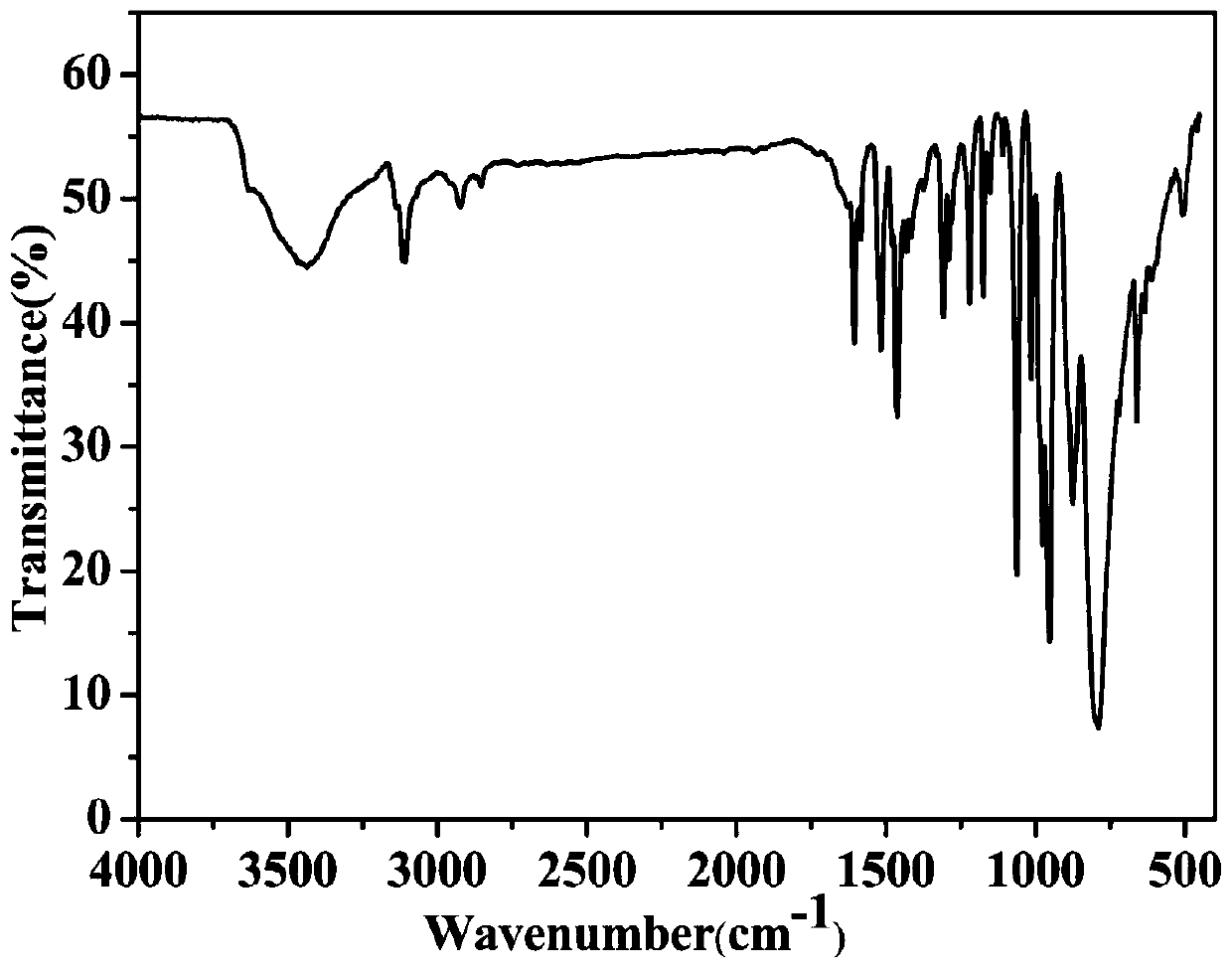
[0025] AgNO 3 (0.1mmol), H 3 PMo 12 O 40The mixture of (0.05mmol), btp (0.2mmol) and 8ml of distilled water was put into a 25ml reactor, stirred for 40min to uniformly mixed, in the stirring process, the pH of the solution was adjusted to 2.1 with HCl, heated at 150°C for five days, and cooled to room temperature After washing, filtering and drying, golden yellow massive crystals were obtained. Yield: 63.2%. Chemical formula of the obtained crystal: Ag 3 PMo 12 C 18 H 17 N 14 O 41.5 ; Elemental analysis: theoretical value: C 8.32, H 0.66, N 7.54; experimental value: C 8.26, H 0.59, N 7.67. Infrared spectrum (cm -1 ): 3440(w), 3120(w), 1609(m), 1527(m), 1460(m), 1310(w), 1222(w), 1170(w), 1065(s), 961(s) ), 877(s), 798(s).
Embodiment 2
[0026] Example 2 Synthesis of polyacid-based Ag(I) coordination polymer
[0027] AgNO 3 (0.1mmol), H 3 PMo 12 O 40 The mixture of (0.04mmol), btp (0.22mmol) and 7ml of distilled water was put into a 25ml reactor, stirred for 35min to uniformly mixed, in the stirring process, the pH of the solution was adjusted to 1.8 with HCl, heated at 140°C for six days, and cooled to room temperature After washing, filtering and drying, golden yellow massive crystals were obtained with a yield of 40.2%.
Embodiment 3
[0028] Example 3 Synthesis of Polyacid Ag(I) Coordination Polymer
[0029] AgNO 3 (0.1mmol), H 3 PMo 12 O 40 The mixture of (0.06mmol), btp (0.19mmol) and 9ml of distilled water was put into a 25ml reactor, stirred for 45min until uniformly mixed, and the pH of the solution was adjusted to 2.3 with HCl during stirring, heated at 160°C for four days, and cooled to room temperature After washing, filtering and drying, golden yellow massive crystals were obtained with a yield of 21.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com