A kind of electrochemical preparation method of 1,3,5-hexatriene
A technology of hexatriene and hexadienedioic acid is applied in the field of electrochemical preparation of 1,3,5-hexatriene, and achieves the effects of low reaction cost, high product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Drugs: Deionized water (18.2MΩ, Merck Milli-QAdvantageA10 ultrapure water system), adiene diacid, potassium hydroxide, potassium sulfate, and sulfuric acid used were bought back and used directly without further purification.
[0022] Electrolytic cell: The electrolytic cell used in this reaction is 50mm 3 The screw port can be sealed without diaphragm Pikes glass electrolytic cell. The electrolysis system is a three-electrode system, in which a Pt sheet (1cm×1cm) is used as the working electrode, a Pt mesh (60 mesh, 1cm×1cm) is used as the counter electrode, and a Hg / HgO (1M KOH) electrode is used as the reference electrode.
[0023] 17.7mg of hexadienedic acid and 0.625ml of 2mol / L KOH ethanol solution (as a supporting electrolyte) were successively added to the beaker and mixed, then added absolute ethanol to make the volume to 25ml. Put the magnet into the beaker, turn on 600rpm and stir for 10min, then add it to the above electrolytic cell. Place the electrolytic...
Embodiment 2-25
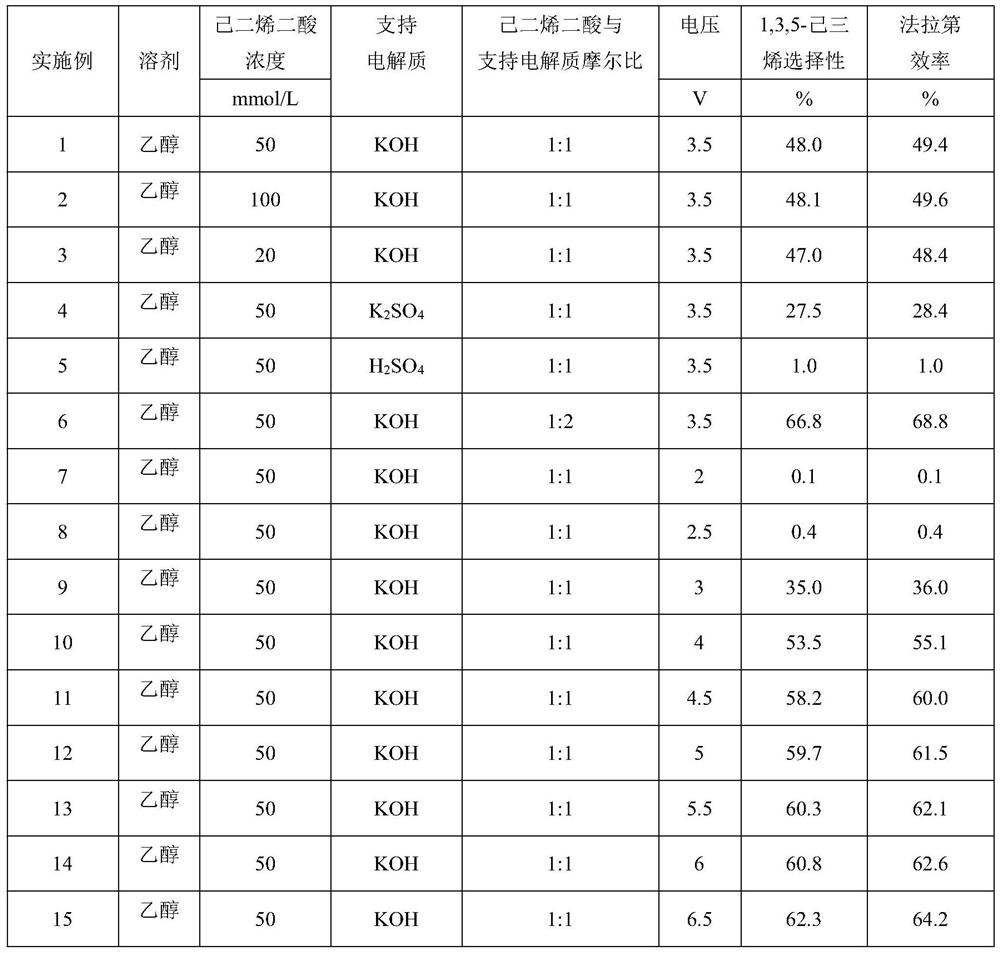
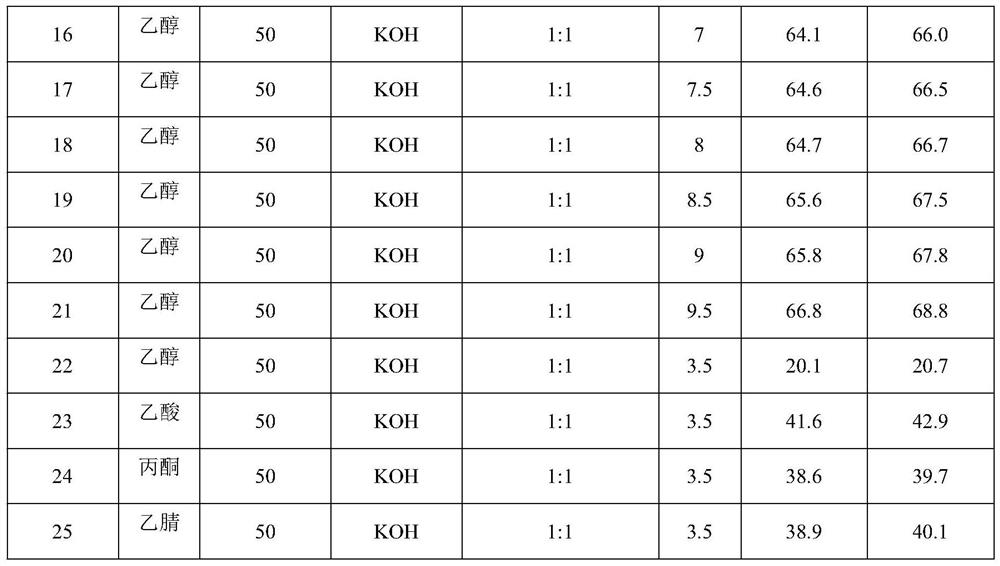
[0026] The influence of the concentration of hexadienedioic acid, solvent type, type of supporting electrolyte, concentration and applied voltage on the selectivity of 1,3,5-hexatriene in hydrocarbon products is shown in Table 1. Wherein embodiment 2-21 and 23-25 use working electrode with embodiment 1, the working electrode that embodiment 22 uses is graphite electrode. Others are the same as embodiment 1.
Embodiment 1-25
[0028]
[0029]
[0030] As can be seen from the data in Table 1: under the condition that the solvent is ethanol and other conditions are constant, the higher the concentration of adienedioic acid, the higher the Faradaic efficiency and the selectivity of 1,3,5-hexatriene. Considering other Factors, generally choose the concentration of adienedioic acid to be 50mmol / L. Under certain other conditions, the supporting electrolyte is alkaline, the faradaic efficiency and 1,3,5-hexatriene selectivity are high, the stronger the alkalinity, the faradaic efficiency and 1,3,5-hexatriene selectivity are higher High; the supporting electrolyte is acidic, the lower the selectivity of 1,3,5-hexatriene; considering other factors, KOH is generally selected, and the molar ratio of it to hexadiene dioic acid is 1:1. Under other conditions, the higher the voltage, the higher the Faradaic efficiency and 1,3,5-hexatriene selectivity; when the voltage is lower than 3V, the Faradaic efficien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com