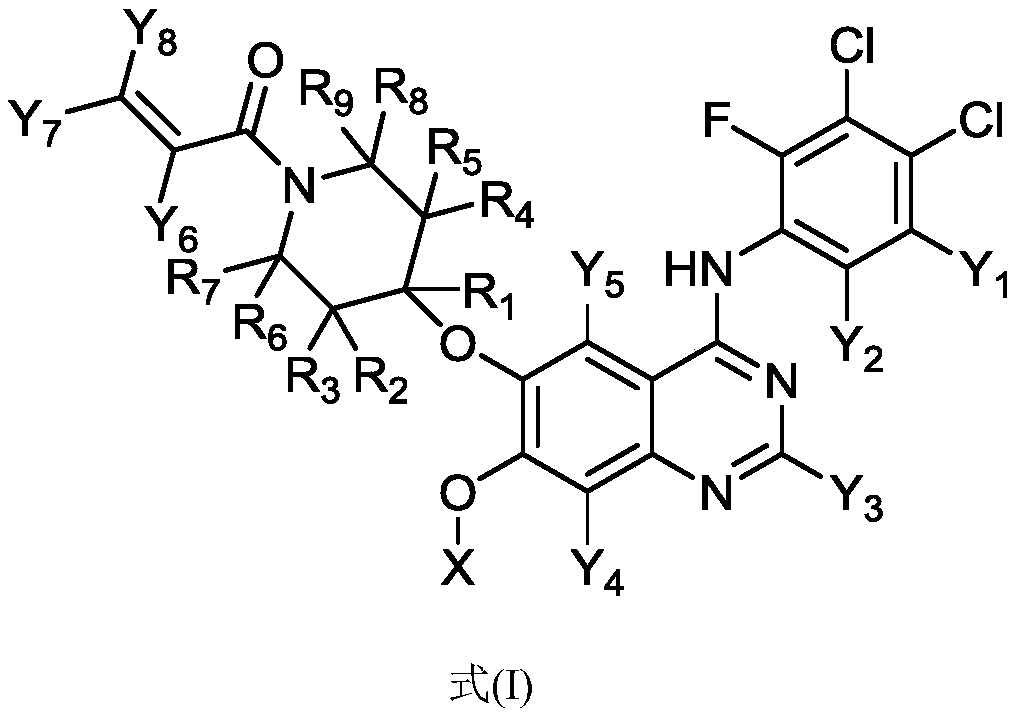
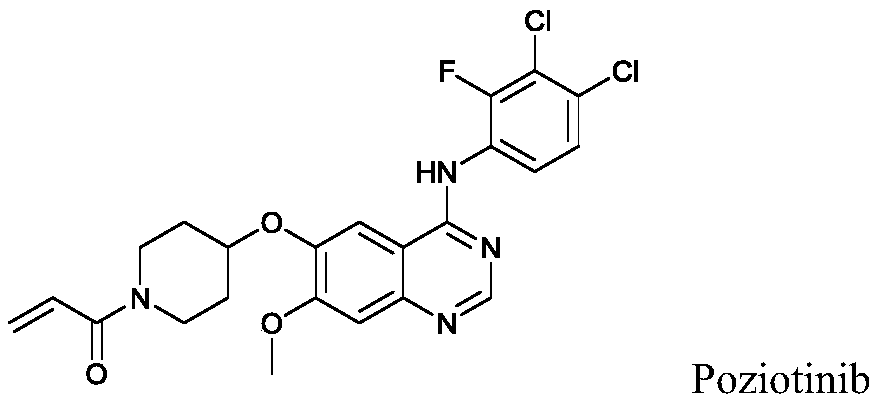
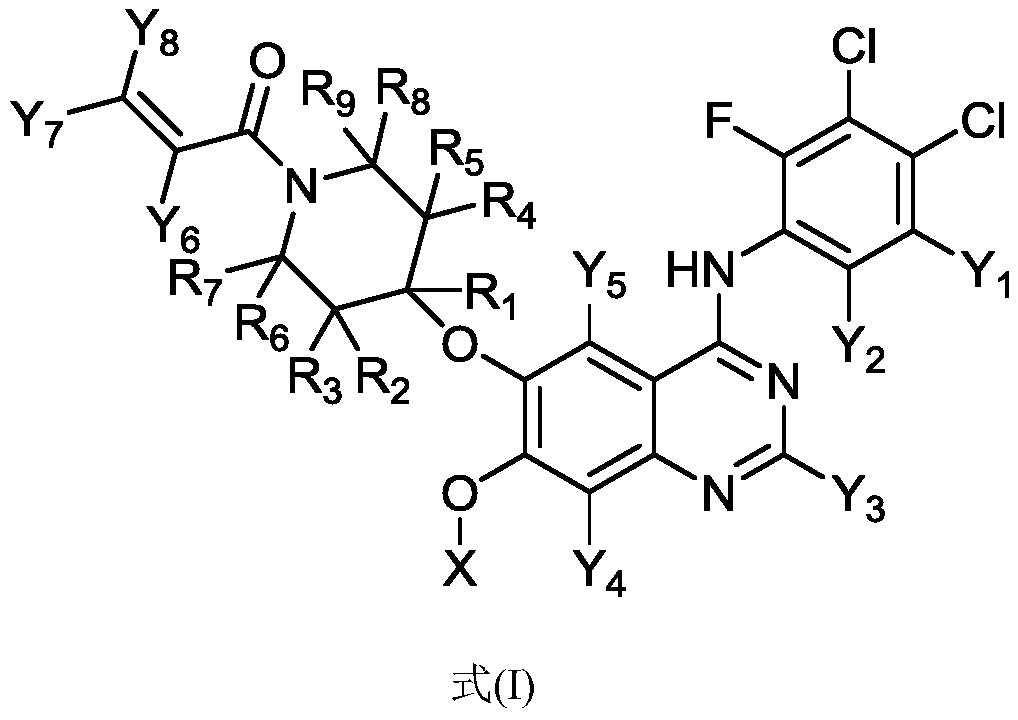
Substituted amino quinazoline compound and pharmaceutical composition and application thereof
A technology of compounds and compositions, applied in the field of medicine, can solve the problems of limited application scope, cost of side effects treatment, poor patient compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found in, eg, Wuts and Greene, Protective Groups in Organic Synthesis, 4th Edition, John Wiley & Sons: New Jersey, (2006), which is incorporated herein by reference in its entirety.
[0107] The reaction can be monitored according to any suitable method known in the art. For example, spectroscopic means such as nuclear magnetic resonance (NMR) spectroscopy (e.g. 1 H or 13 C), infrared (IR) spectroscopy, spectrophotometry (e.g., UV-visible), mass spectroscopy (MS)) or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC) Product formation was monitored.
[0108] The following general preparation r...
Embodiment 1
[0162] Example 1 tert-butyl 4-(p-toluenesulfonyloxy)piperidine-1-carboxylate-3,3,5,5-d 4 Preparation of (Intermediate A-1) prepare.
[0163]
[0164] Synthesize using the following route:
[0165]
[0166] Synthesis of Step 1 Compound 2
[0167] TBU was added to compound 1 (10 g, 50.2 mmol) in deuterated chloroform (100 mL), and the reaction solution was stirred at room temperature for 24 hrs. The reaction solution was neutralized with 1M dilute hydrochloric acid, then extracted with dichloromethane, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 10.02 g of a yellow solid, which was directly used in the next step.
[0168] Step 2 Synthesis of Compound 3
[0169] Under ice-cooling, sodium borohydride (200 mg) was added in batches to compound 2 (2.0 g) in anhydrous methanol (10 mL), and the mixture was reacted at room temperature for 4 hrs. The reaction was quenched...
Embodiment 2
[0172] Example 2 Preparation of tert-butyl 4-(p-toluenesulfonyloxy)piperidine-1-carboxylate-4-d (Intermediate A-2).
[0173]
[0174] Synthesize using the following route:
[0175]
[0176] Step 1 Synthesis of Compound 4
[0177] Under ice-cooling, deuterated sodium borohydride (300 mg) was added in batches to compound 1 (2.0 g) in deuterated methanol (15 mL), and the reaction was carried out at room temperature for 4 hrs. The reaction was quenched by adding saturated ammonium chloride solution, and the deuterated methanol was removed under reduced pressure. Extracted with ethyl acetate (50 mL x 3), combined the organic layers, washed with saturated brine, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain an oil that was directly used in the next reaction.
[0178] Step 2 Synthesis of intermediate compound A-2
[0179] Under ice-cooling, TsCl was added to the above compound 4, DMAP (75mg) and T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com