Chondroitin sulfate synthase and encoding gene and application thereof
A technology of chondroitin sulfate and chondroitin sulfate, applied in the direction of enzymes, enzymes, transferases, etc., can solve the problems of limited types and limited research and development of chondroitin sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
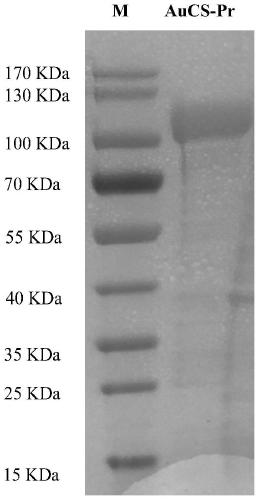
[0052] Embodiment 1. Preparation of recombinant chondroitin sulfate synthase AuCS
[0053] (1) Construction of expression strains
[0054] The nucleotide sequence of chondroitin sulfate synthase AuCS is shown in SEQ IND NO.1, and the amino acid sequence is shown in SEQ IND NO.2. The nucleotide sequence of chondroitin sulfate synthase AuCS was artificially synthesized, and the above nucleotide sequence was inserted into the plasmid vector pET28a(+) to construct the recombinant plasmid pET28a(+)-His-AuCS. Siri Corporation assisted in the completion. The recombinant plasmid pET28a(+)-His-AuCS constructed by Nanjing GenScript Company was transformed into Escherichia coli BL21(DE3) competent cells (purchased from Beijing Tiangen Biochemical Technology Co., Ltd.), and kanamycin (100 μg / mL) cultured on LB solid medium at 37°C for 12 hours, screened transformants, and used Escherichia coli BL21(DE3) competent cells that had not been transformed with recombinant plasmids as a negati...
Embodiment 2
[0061] Example 2. Verification of the activity of chondroitin sulfate synthase AuCS
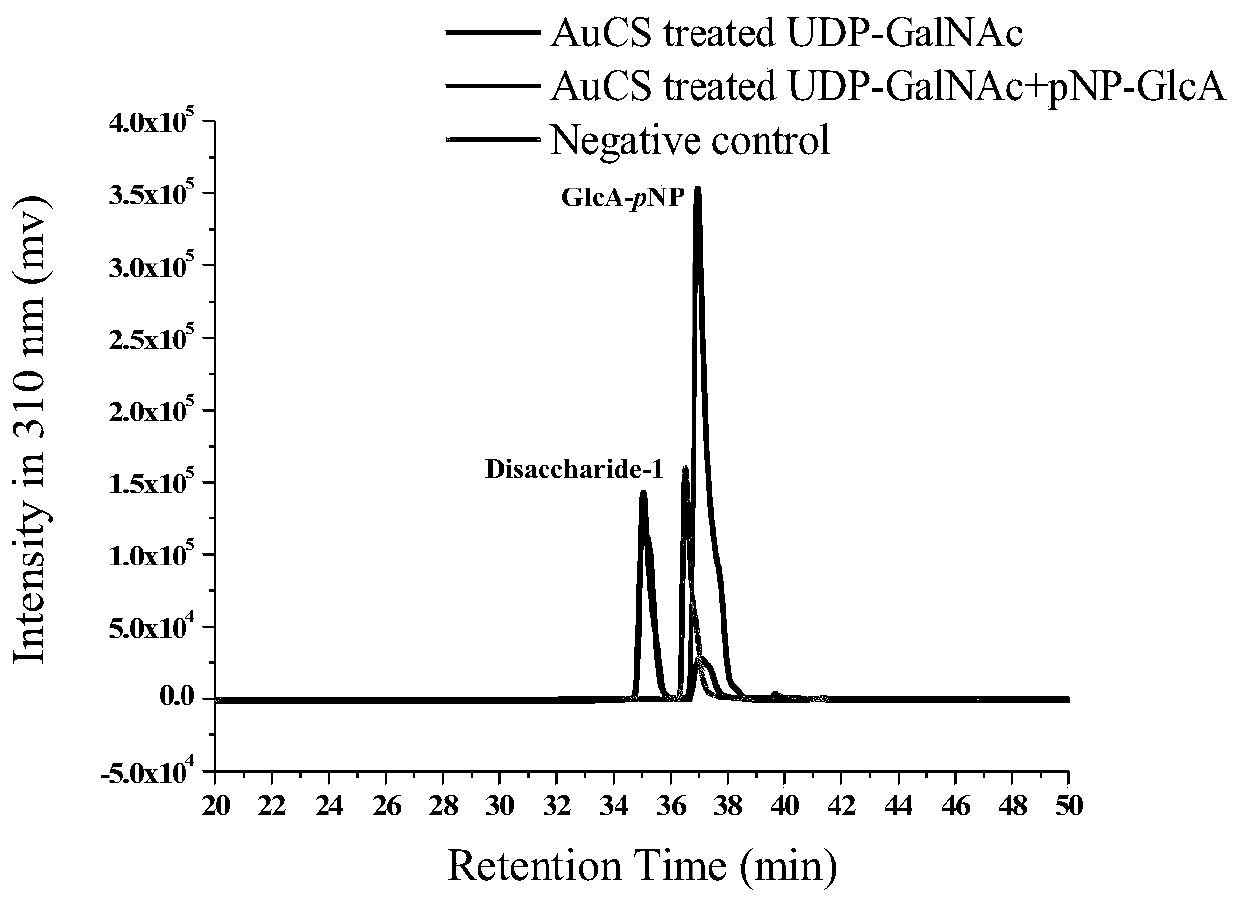
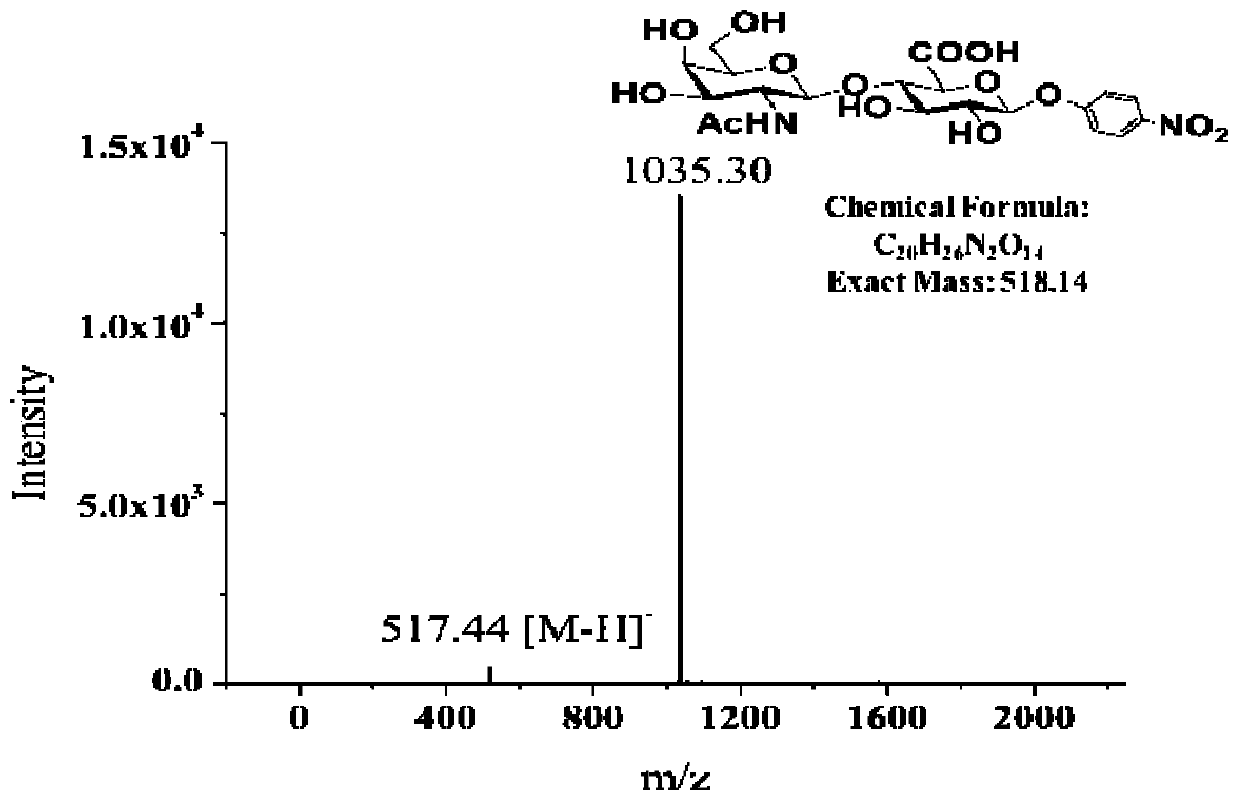
[0062] (1) Verification of GalNAc transferase activity of chondroitin sulfate synthase AuCS
[0063] Use commercial GlcA-pNP (final concentration is 0.2mM) as acceptor substrate, UDP-GalNAc (final concentration is 0.3mM) reacts as donor substrate, and reaction system is as shown in table 2; React in a water bath at ℃ for 4 hours, heat in boiling water for 5 minutes to inactivate the enzyme to terminate the reaction, filter the reaction solution through a 0.22 μm filter membrane, and perform liquid phase detection according to the method described in Table 1. There is specific absorption at the detection wavelength, and the flow rate of the mobile phase is 0.5 mL / min.
[0064] Table 2 Reaction system for verification of GalNAc transferase activity of AuCS
[0065]
[0066] Note: AuCS is the chondroitin sulfate synthase AuCS prepared in Example 1.
[0067] The liquid phase test results ar...
Embodiment 3
[0080] Example 3 Properties of Chondroitin Sulfate Synthase AuCS
[0081] (1) Determination of the optimal reaction pH of the in vitro reaction of AuCS
[0082] The reaction system is shown in Table 2 except for the pH of the buffer, and the Tris-HCl buffer was replaced with Tris-HCl / PBS / CH with different pH values. 3 For COONa buffer, set 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.3, 5.9, 6.4, 7.0, 7.5, 8.0, 8.5, a total of 13 pH gradient points, and each gradient has three parallel groups. Each reaction system was reacted in a water bath at 25°C for 4 hours, and heated in boiling water for 5 minutes to inactivate the enzyme to terminate the reaction.
[0083] The result is as Figure 6 As shown, the results indicated that the enzyme was active in a wide pH range (5.0-8.5), and the optimum pH for the reaction was 5.0-6.5.
[0084] (2) Determination of the optimal metal ion for the in vitro reaction of AuCS
[0085] The reaction system is shown in Table 2 except for metal ions, Mn 2+ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com