Preparation technology of diazomethane
A preparation process, diazomethane technology, applied in the preparation of organic compounds, preparation of urea derivatives, organic chemistry, etc., can solve the problem of high cost of diazomethane equipment, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation process of the present application does not use expensive water removal membranes or equipment, and can obtain a diazomethane solution with extremely low water content at a relatively low cost, which can directly react with water-sensitive materials or systems;
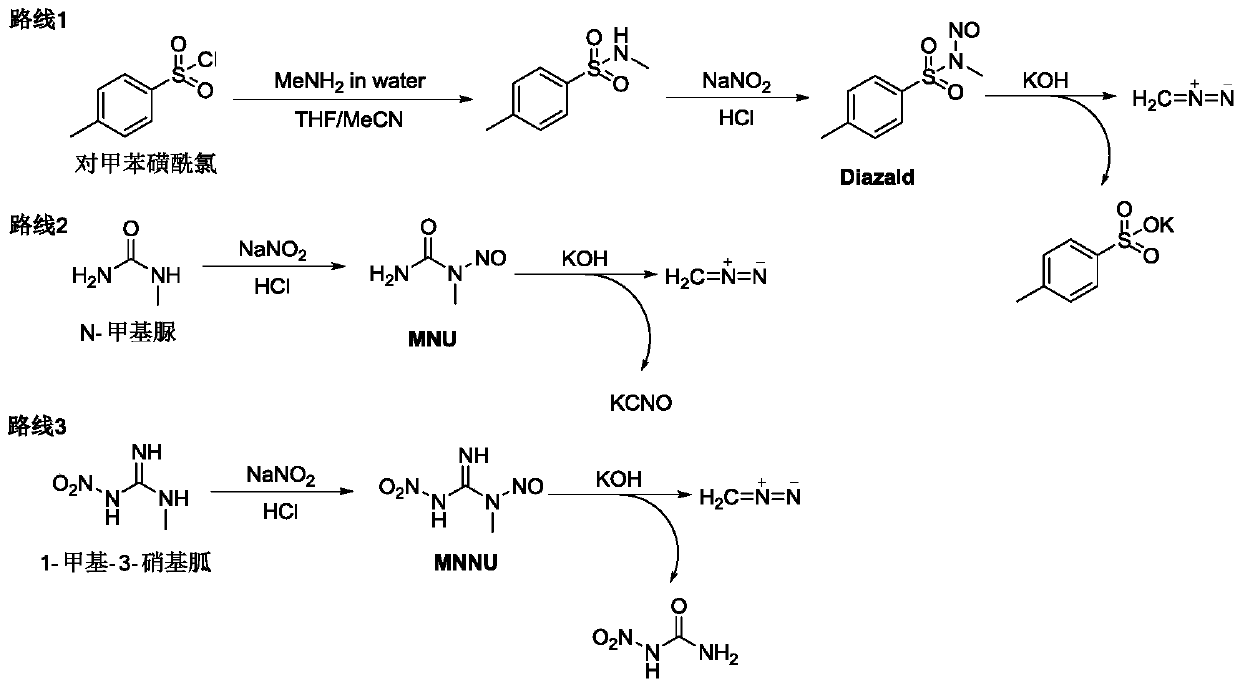
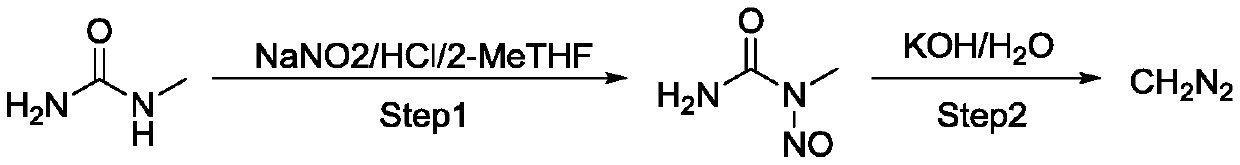
[0032] Using low-cost, non-toxic N-methylurea as the starting material, the MNU obtained by continuous preparation can be directly prepared in the second step of continuous diazomethane without separation and purification, reducing the exposure to carcinogenic and allergenic materials MNU risk, and compared with most reactions using Diazald as the diazomethane precursor, the cost is lower and the three wastes are less;
[0033] The unique properties of continuous equipment (small reaction system and much higher heat exchange rate than batch equipment) make the reaction conditions more severe, but the safety is higher than that of batch reactions;
[0034] The unique properties of the continuous ...
Embodiment 1
[0047] Configure the feeding system A: Add 100g of N-methylurea to the feeding bottle A, add 100ml of water, slowly add 266g of 37% hydrochloric acid, and stir until dissolved.
[0048] Configure feeding system B: Add 1750mL 2-Me-THF into feeding bottle B.
[0049] Configure feeding system C: Add 140g of sodium nitrite to feeding bottle C, add 400ml of water, and stir until dissolved.
[0050] Configure feeding system D: Add 600mL 2-Me-THF into feeding bottle D.
[0051] Configure the mixing system E: Add potassium bicarbonate to the mixing bottle E, add 654ml of water, and stir until dissolved.
[0052] Equipment preparation: Add about 500ml of tap water to the first extraction column to half the column height; add about 200ml potassium bicarbonate aqueous solution to the second extraction column to 1 / 4 of the column height.
[0053] Fill the 200ml first PTFE coil reactor with tap water to 23-27°C (target temperature 25°C), after the temperature has stabilized for 10 minute...
Embodiment 2
[0065] The difference from Example 1 is that during the process of liquid separation and water removal, the upper yellow organic phase overflowed into a CSTR at -50°C and stirred for 40 minutes to freeze and remove water. The water solidified into ice at a low temperature to separate from the system. The organic solution of methane overflows through the overflow port with filter screen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com