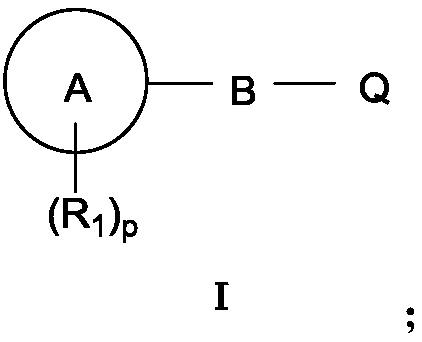
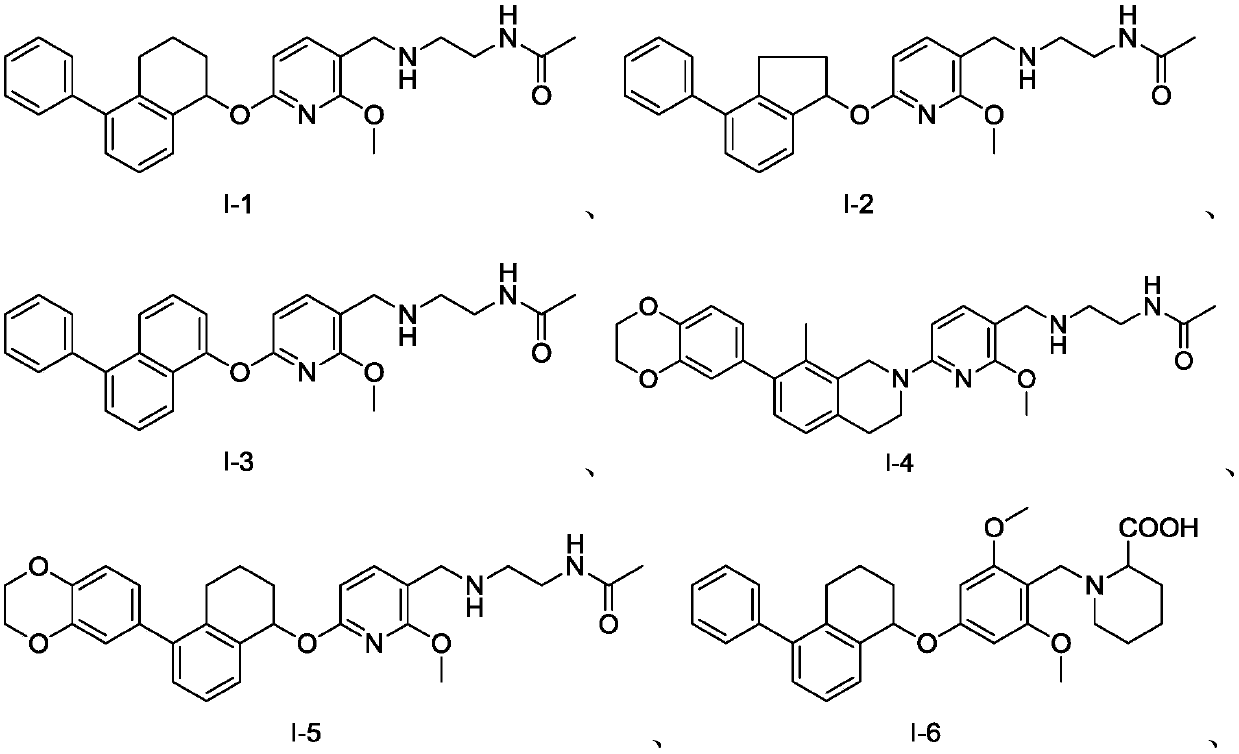
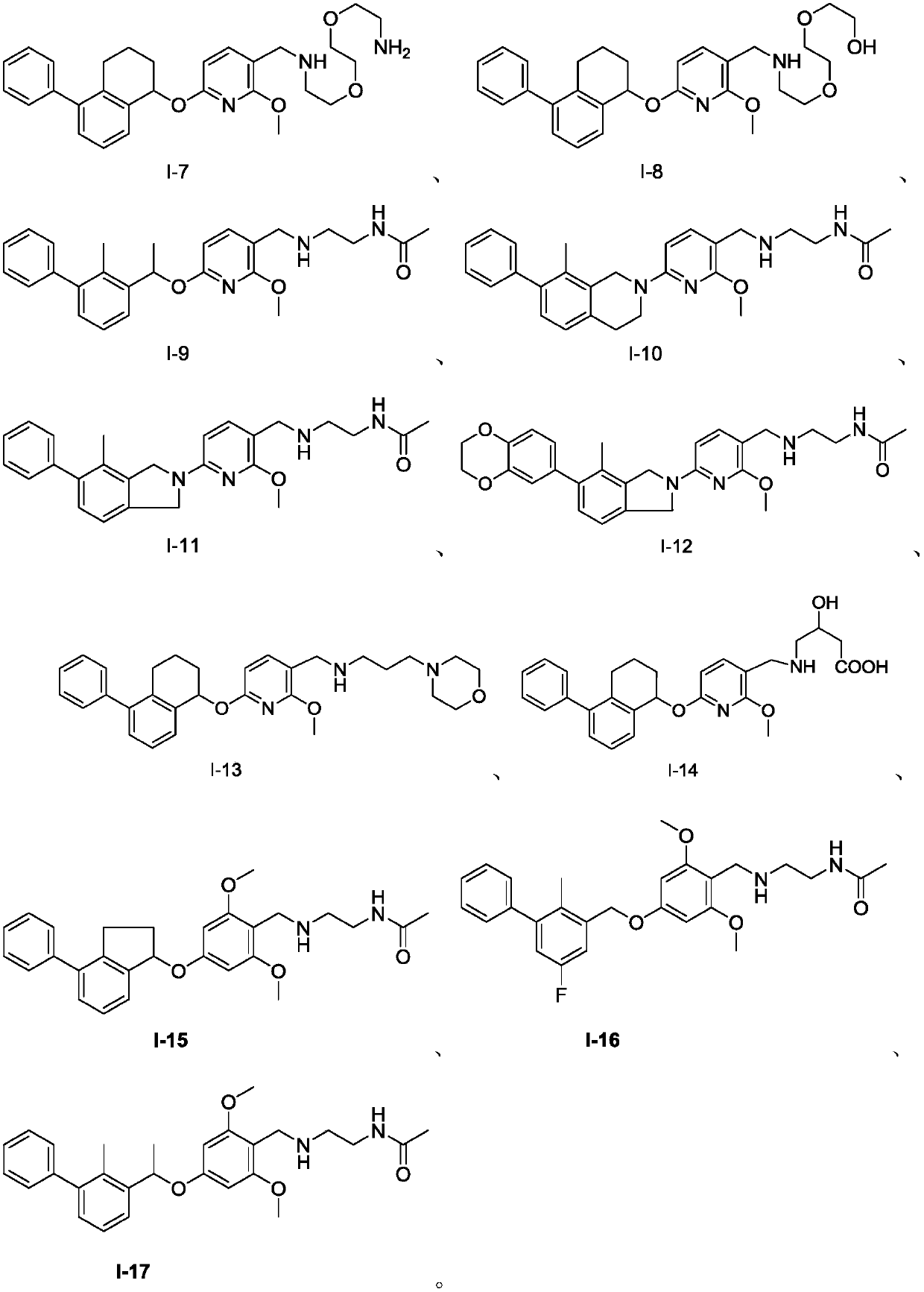
Aromatic ring-containing compound and application thereof
A compound and aromatic ring technology, applied in the field of aromatic ring-containing compounds, can solve the problems of intravenous injection, poor therapeutic activity of solid tumors, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The preparation of embodiment 1 compound I-1
[0108] synthetic route:
[0109]
[0110] Sodium cyanoborohydride (314mg, 5mmol), compound I-1-6 (360mg, 1mmol) and compound I-1-7 (410mg, 4mmol) were mixed in DMF (40mL) and acetic acid (1.6mL), in Stir overnight at room temperature, and monitor with TLC until the reaction is complete. After post-treatment, the obtained crude product is purified by preparative LC / MS to obtain compound I-1, and its purity is 98.0% as assessed by LCMS analysis.
[0111] LC-MS: m / z, [M+H] + =446.
Embodiment 2
[0112] The preparation of embodiment 2 compound I-15
[0113] synthetic route:
[0114]
[0115] Step 1: Synthesis of compound I-15B
[0116] Compound I-15A (10.5g, 50mmol) was dissolved in methanol (100ml), cooled in an ice bath to below 5 degrees Celsius, and then sodium borohydride was added in batches. After the addition, it was reacted at room temperature for two hours. TLC showed that the reaction was complete. Add The reaction was quenched with 10 ml of water, and after the methanol was spun off under reduced pressure, saturated ammonium chloride solution (250 ml) was added. Extracted with ethyl acetate (150mL × 3), the combined organic layer was washed with brine, then dried over anhydrous sodium sulfate, filtered to remove the desiccant, precipitated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / Ethyl acetate=10 / 1), to obtain compound I-15B (9.2 g, light yellow liquid), yield: 86.8%.
[0117] The secon...
Embodiment 3
[0126] The preparation of embodiment 3 compound I-16
[0127] synthetic route:
[0128]
[0129] Step 1: Synthesis of Compound I-16B
[0130] Compound I-16A (15.5g, 100mmol) was dissolved in methanol (100ml), and then 2ml of concentrated sulfuric acid was added, and the resulting reaction solution was reacted overnight at 60 degrees Celsius. After TLC showed that the reaction was over, it was cooled to room temperature, and the methanol was spun off. Saturated ammonium chloride solution (250ml) was added. Extracted with ethyl acetate (150mL × 3), the combined organic layer was washed with brine, then dried over anhydrous sodium sulfate, filtered to remove the desiccant, precipitated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / Ethyl acetate=10 / 1 (volume ratio V / V)) to obtain compound I-16B (15 g, light yellow liquid), yield: 89%.
[0131] MS m / z(ESI):169[M+23].
[0132] The second step: synthesis of compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com