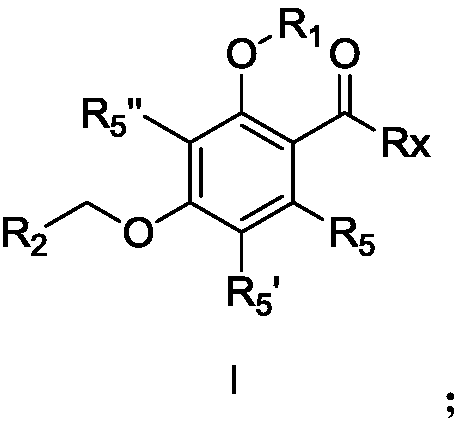
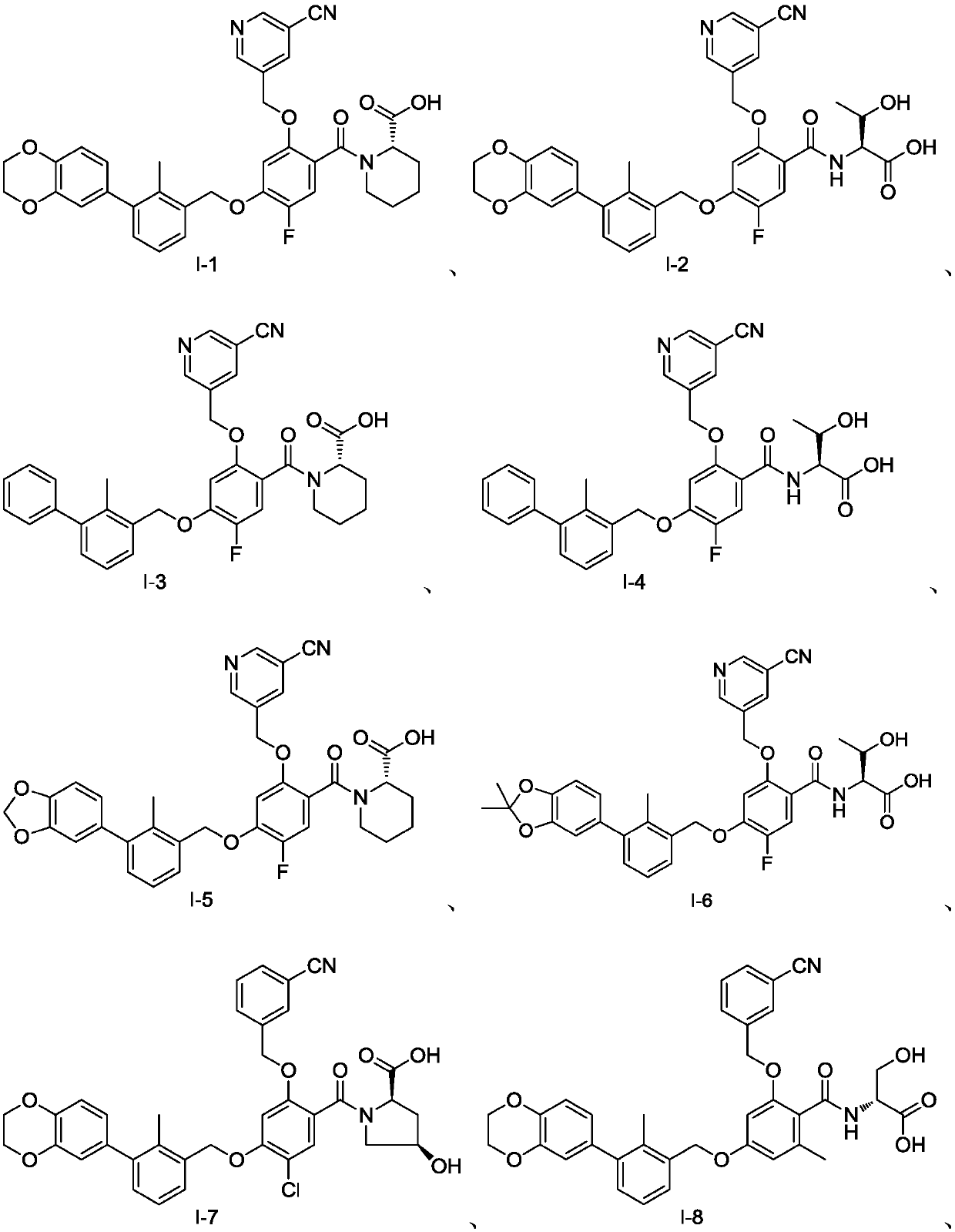
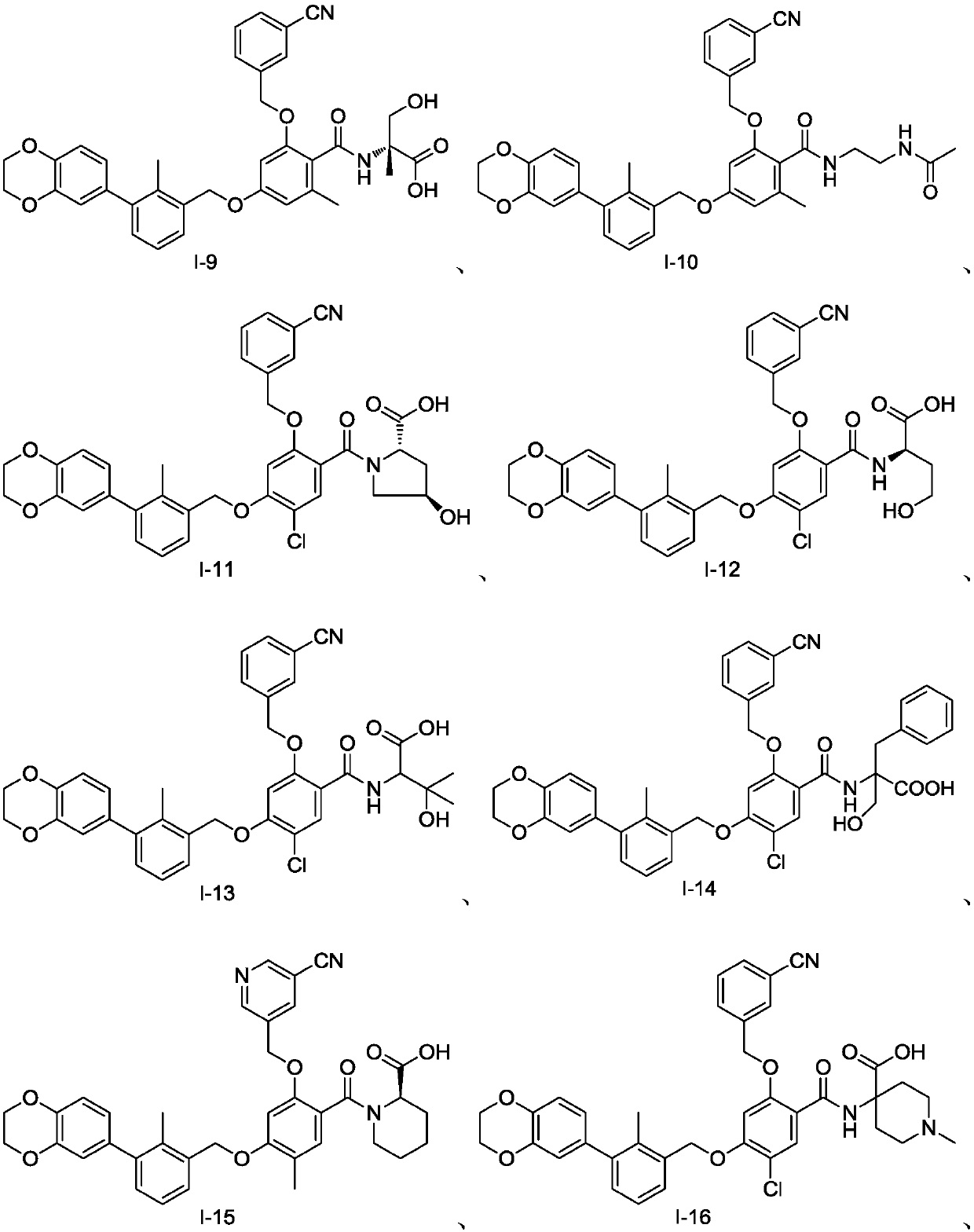
Substituted phenyl compound and application thereof
A compound, phenyl technology, applied in the field of substituted phenyl compounds, can solve problems such as low bioavailability, need for intravenous injection, and poor therapeutic activity for solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Preparation of Example 1 Compound I-2
[0123]
[0124] (1) Preparation of compound I-2-2
[0125]
[0126] Compound I-2-1 (4.3 g, 31.13 mmol), 1-chloromethyl-4-fluoro-1,4-diazobicyclo 2.2.2 octane bis(tetrafluoroborate) salt (15 g, 42.34 mmol) was added to a 250mL single-neck bottle, acetonitrile (60mL) was added to react at room temperature for 5 days, the reaction system was spin-dried, and column chromatography gave compound I-2-2, yield 2.8g, yield 28.8%.
[0127] 1 H NMR (500MHz, CDCl 3 ): δ=11.22(s, 1H), 9.66(s, 1H), 7.24(d, J=5.0Hz, 1H), 6.58(d, J=5.0Hz, 1H).
[0128] (2) Preparation of compound I-2-4
[0129]
[0130] Compound I-2-3 (2.0 g, 14.91 mmol) was added to a 50 mL single-neck flask, thionyl chloride (6.5 mL) and dichloromethane (20 mL) were added to react at room temperature for 3 h, TLC detected that the reaction was complete, and the reaction system was Spin to dry and oil pump to dry to obtain compound I-2-4, the yield is 1.8 g, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com