Heavy metal stabilizer and preparation method and use thereof
A heavy metal stabilizer and cross-linking agent technology, applied in chemical instruments and methods, water pollutants, flocculation/sedimentation water/sewage treatment, etc., can solve the problems of poor Zn2+ removal ability and achieve good removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
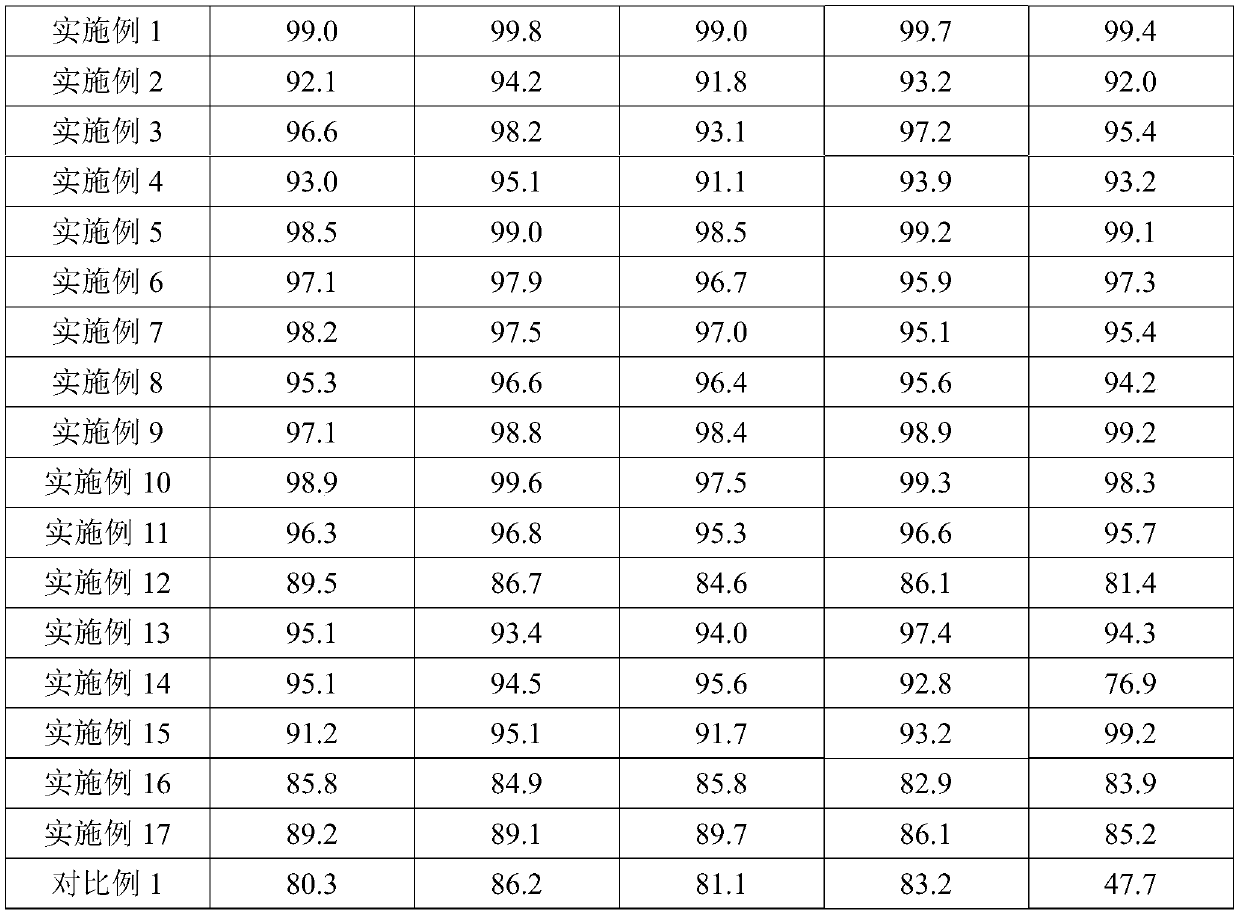
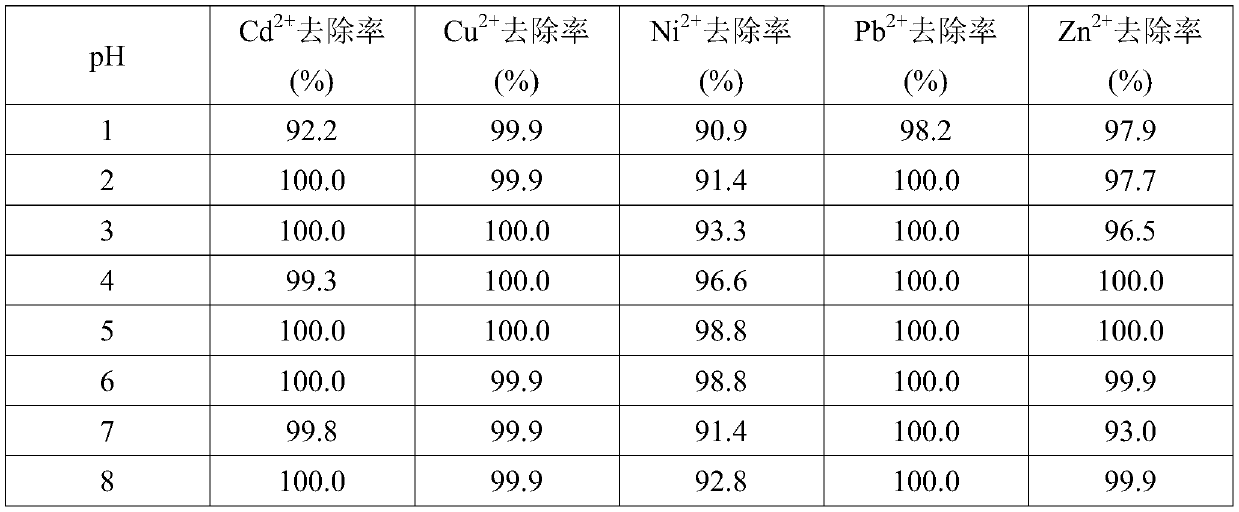
Examples
Embodiment 1
[0043] The present embodiment provides a kind of heavy metal stabilizer, and its preparation method is as follows:
[0044] (1) Dissolve ethylenediamine (0.5mol) in deionized water at 20°C to form an aqueous solution of ethylenediamine, stir until the temperature returns to normal temperature, and set aside;
[0045] Weigh solid sodium hydroxide (1.5mol) and dissolve it in water to obtain 5mol / L lye, add it into the above-mentioned ethylenediamine aqueous solution through a constant pressure dropping funnel, after the two are fully mixed, use a constant pressure dropping funnel to slowly Add carbon disulfide (0.9mol) dropwise, and control the temperature below 30°C during the dropwise addition;
[0046] After the dropwise addition, the temperature of the system was controlled by an oil bath to 40°C, and the reaction was refluxed for 4 hours to obtain a dithiocarbamate solution;
[0047] (2) Ferrous chloride (0.15mol) solution is slowly dripped into the dithiocarbamate solutio...
Embodiment 2
[0050] The present embodiment provides a kind of heavy metal stabilizer, and its preparation method is as follows:
[0051] (1) Dissolve ethylenediamine (0.5mol) in deionized water at 20°C to form an aqueous solution of ethylenediamine, stir until the temperature returns to normal temperature, and set aside;
[0052] Weigh solid sodium hydroxide (1.5mol) and dissolve it in water to obtain 5mol / L lye, add it into the above-mentioned ethylenediamine aqueous solution through a constant pressure dropping funnel, after the two are fully mixed, use a constant pressure dropping funnel to slowly Add carbon disulfide (0.5mol) dropwise, and control the temperature below 30°C during the dropwise addition;
[0053] After the dropwise addition, the temperature of the system was controlled to 20°C by an oil bath, and the reaction was refluxed for 12 hours to obtain a dithiocarbamate solution;
[0054] (2) Ferrous chloride (0.1mol) solution is slowly dripped into the dithiocarbamate solutio...
Embodiment 3
[0057] The present embodiment provides a kind of heavy metal stabilizer, and its preparation method is as follows:
[0058] (1) Dissolve ethylenediamine (0.5mol) in deionized water at 20°C to form an aqueous solution of ethylenediamine, stir until the temperature returns to normal temperature, and set aside;
[0059] Weigh solid sodium hydroxide (1.5mol) and dissolve it in water to obtain 5mol / L lye, add it into the above-mentioned ethylenediamine aqueous solution through a constant pressure dropping funnel, after the two are fully mixed, use a constant pressure dropping funnel to slowly Add carbon disulfide (2mol) dropwise, and control the temperature below 30°C during the dropwise addition;
[0060] After the dropwise addition, the temperature of the system was controlled by an oil bath to 30°C, and the reaction was refluxed for 8 hours to obtain a dithiocarbamate solution;
[0061] (2) Ferrous chloride (0.3mol) solution is slowly dripped into the dithiocarbamate solution o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com