Isomer impurities in bulk pharmaceutical chemicals of enalapril maleate and synthesis method of isomer impurities
A technology of enalapril and enantiomers, applied in the field of isomer impurities and their synthesis, can solve the problems of teratogenicity, no disclosure of enalapril maleate enantiomeric impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
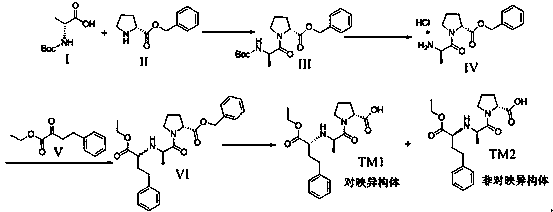
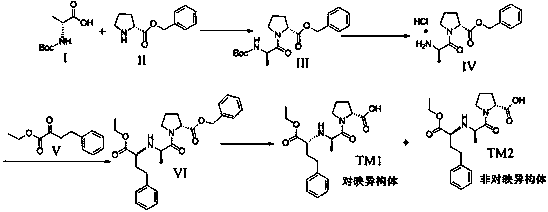
[0052] Example 1: Compound I and Compound II are condensed to prepare Compound III
[0053] Dissolve 30g of N-tert-butoxycarbonyl-D-alanine (I) and 33g of D-proline benzyl ester in 300ml of dichloromethane, then add 32g of HOBT and 41g of N,N-diiso Propylamine, stir, cool down to 0~10 ℃, add 46g EDCI in batches, after adding, keep warm for 1 hour, raise to room temperature to react to complete, add water to wash, separate liquid, the organic layer is dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 68g The oily substance was purified by column chromatography to obtain compound III 37g oily substance, the yield was 62%.
Embodiment 2
[0054] Example 2: Compound III was removed from Boc to prepare compound IV
[0055] Dissolve 37 g of compound III in 370 ml of ethyl acetate, stir, add 74 ml of ethyl acetate hydrochloride dropwise, stir at room temperature until complete deprotection, precipitate a solid, filter, wash with ethyl acetate, and dry to obtain product IV 17.9 g as a white solid. The rate was 58.2%.
Embodiment 3
[0056] Example 3: Reductive amination of compound IV with ethyl 2-oxo-4-phenylbutanoate (V) to prepare compound IV
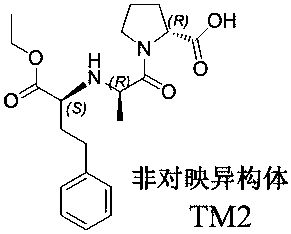
[0057] Add 17g of product IV to 170ml of absolute ethanol, stir, add 8.5g of anhydrous sodium acetate and 13.6g of ethyl 2-oxo-4-phenylbutyrate (V), cool to 0~5℃, dropwise A solution of 6.8 g of sodium cyanoborohydride dissolved in 34 ml of absolute ethanol was added, and the dropwise addition was completed. After the reaction was incubated for 1 hour, the reaction was returned to room temperature to complete the reaction. After the reaction was completed, the ethanol was concentrated under reduced pressure, 200 ml of dichloromethane was added, washed with 100 ml of saturated sodium bicarbonate solution, and concentrated under reduced pressure to obtain a crude oily product, which was purified by dichloromethane:methanol 10:1 column chromatography to obtain compound VI 17.8g , the yield is 70.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com