Selenotyrosine translation system and application thereof
A technology of tyrosine and selenogenesis, applied in the field of biochemistry, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
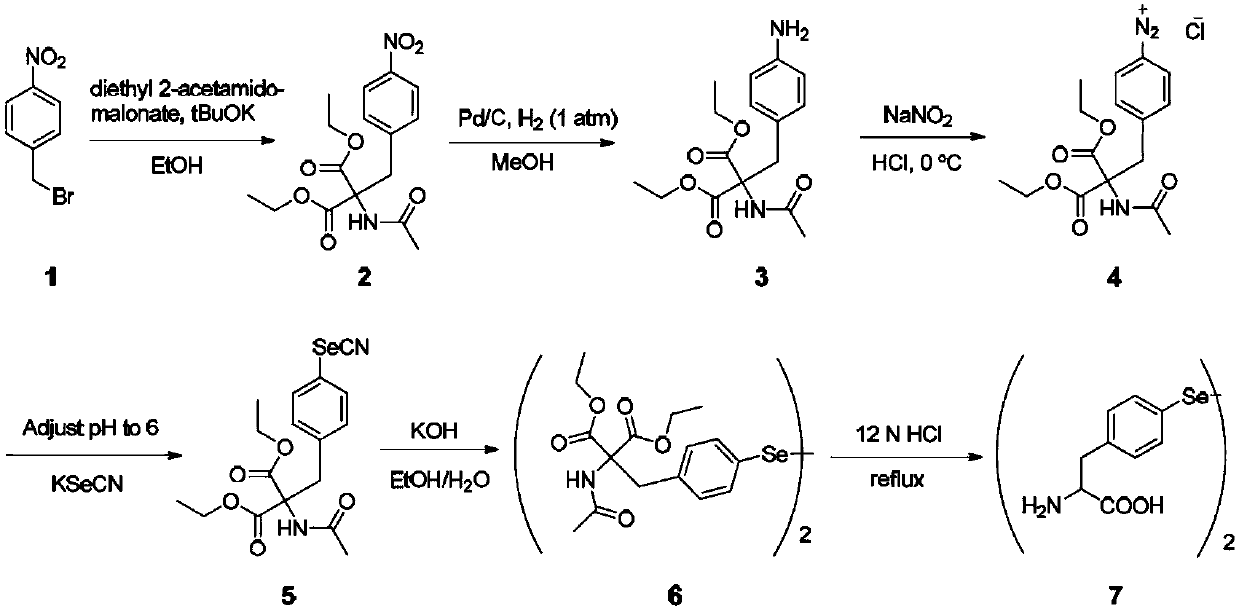
[0089] Embodiment 1: the chemical synthesis of selenotyrosine (SeHF) ( figure 1 )
[0090] Step 1: Synthesis of diethyl 2-acetamido-2-(4-nitrobenzyl)malonate (compound 2)
[0091] A mixture of 1-(bromomethyl)-4-nitrobenzene (1.0 g, 1 eq), diethyl 2-acetamidomalonate (1.2 g, 1.2 eq) and tBuOK (0.63 g, 1.2 eq) Place in a round bottom flask containing 20 mL of EtOH and monitor reflux by TLC overnight. After the reaction was complete, the mixture was cooled to room temperature, filtered and washed with EtOH. The residue was subjected to flash chromatography on silica gel using ethyl acetate / hexane (1:1 mix) as eluent to afford the title compound (1.23 g, 75.5% yield) as a white solid.
[0092] 1H NMR (500MHz, CDCl3) δ8.12(d, 2H), 7.18(d, 2H), 6.56(s, 1H), 4.28(q, 4H), 3.77(s, 2H), 2.04(s, 3H) , 1.3(t,6H).13C NMR(CDCl3)δ169.40, 167.05, 147.27, 143.15, 130.72, 123.46, 66.86, 63.02, 37.59, 23.02, 14.01.
[0093] Step 2: Synthesis of diethyl 2-acetylamino-2-(4-aminobenzyl)malona...
Embodiment 2
[0103] Example 2: Evolution of SeHF-specific aminoacyl-tRNA synthetases
[0104] In order to site-specifically insert SeHF into the gene, it is necessary to introduce an aminoacyl-tRNA synthetase / tRNA orthogonal pair in the E.coli host cell used. Aminoacyl tRNA (MjtRNA Tyr CUA) / tyrosyl tRNA synthetase (MjYRS, wild type, its amino acid sequence is SEQ ID NO: 2) pair. The MjYRS mutation library was constructed in the kanamycin-resistant pBK plasmid (purchased from the laboratory of Peter G. Schultz, Scripps Research Institute, USA), and located between the promoter and terminator of E. coli glutamine synthetase on the plasmid. The synthetic enzyme mutation library used is the pBk-lib-iw1 library, and the construction method of the mutation library is: select 6 sites (Tyr32, Leu65, Phe108, Gln109, Asp158, and Leu162) on the MjYRS gene and introduce NNK mutation ( N=A+T+C+G; K=T+G), the other 6 sites (Ile63, Ala67, His70, Tyr114, Ile159, Val164) were either randomly mutated to G...
Embodiment 3
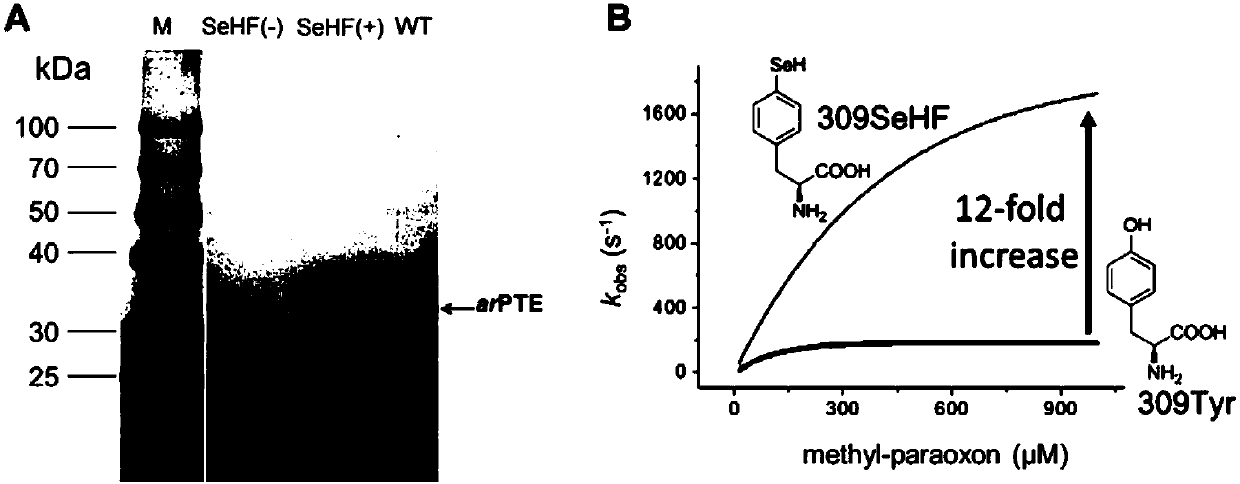
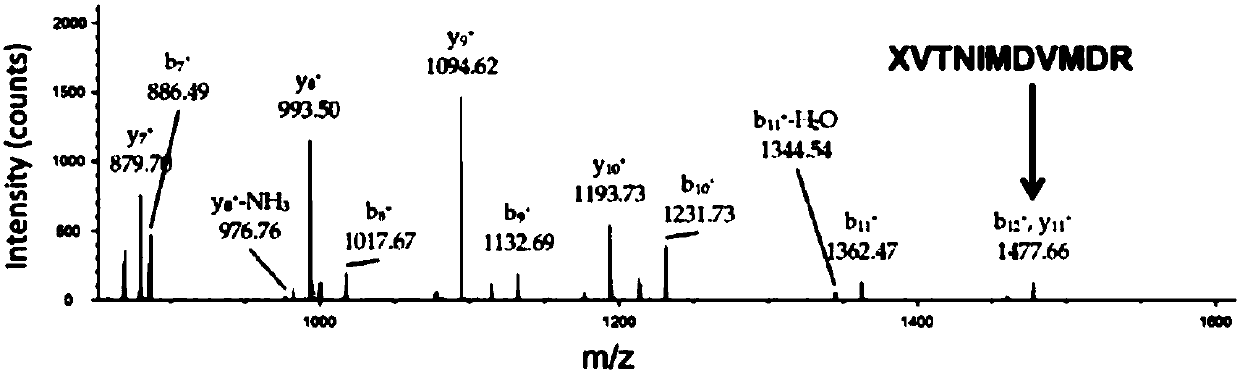
[0108] Example 3: Expression of SeHF-arPTE and identification by mass spectrometry
[0109] To determine the efficiency and fidelity of SeHF incorporation into proteins, we replaced Tyr309 with an amber stop codon in the C-terminal His6-tagged arPTE. In SeHFRS, MjtRNA Tyr CUA Under the condition of co-existing with 0.5mM SeHF, arPTE was expressed by Escherichia coli, and no SeHF was added as a negative control.
[0110] The specific steps are: construct the orthogonal tRNA (SEQ ID NO: 1) and the screened nucleotide sequence encoding SeHFRS (SEQ ID NO: 3) into the pEVOL vector (purchased from Peter G. ); the nucleotide sequence (SEQ ID NO: 5) encoding arPTE was constructed on the pET-22b vector (purchased from Novagen Company), and was designed by using TransStartFastPfu (purchased from Quanshijin Company) DNA polymerase and primers by PCR The Y309TAG mutation was introduced in arPTE-pET22b. The two plasmids were co-transformed into BL21(DE3) cells (purchased from Quanshiji...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com