Micro-molecular fluorescent probe with CS as fluorophore, preparation method and application thereof
A fluorescent probe, small molecule technology, applied in fluorescence/phosphorescence, chemical instruments and methods, material analysis by optical means, etc. The effect of application prospect, simple synthesis method and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of Small Molecule Fluorescent Probes
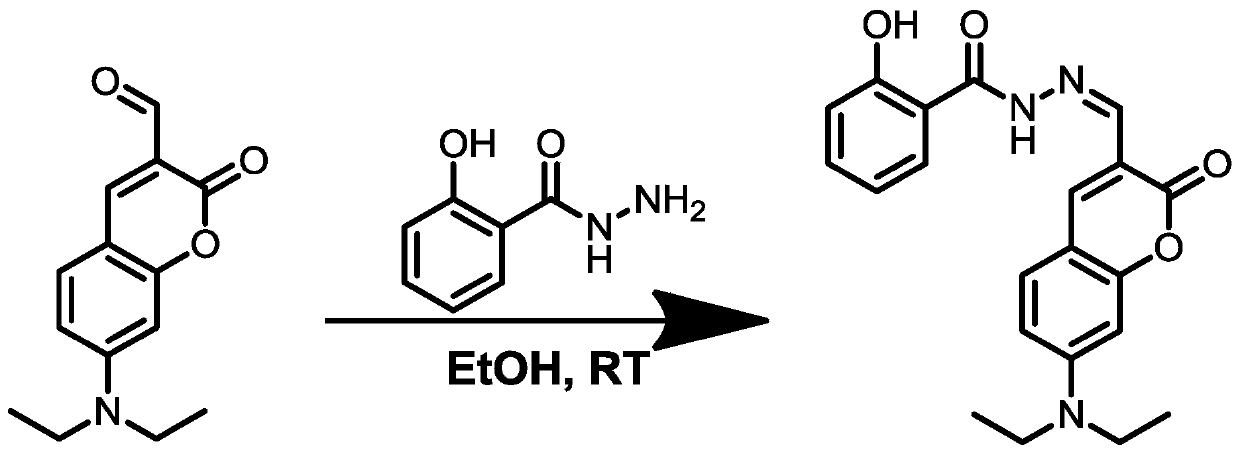
[0037] Synthesis of small molecule fluorescent probes with CS as fluorophore: Dissolve 7-(diethylamino)coumarin-3-carbaldehyde (500mg, 2.04mmol) in 30mL ethanol solution, and add salicylic acid at room temperature Hydrazide (310mg, 1.83mmol) was stirred, stirred at room temperature for 20h, and a yellow solid was obtained, which was washed with ethanol and dried. The small molecule fluorescent probe can be recrystallized with absolute ethanol to obtain a sample with higher purity, and the yield of the final sample is: 80%. The route diagram for synthesizing the small molecule fluorescent probe is shown in figure 1 as shown, figure 1 Represents the roadmap for the synthesis of small molecule fluorescent probes, where EtOH is ethanol and RT is room temperature.
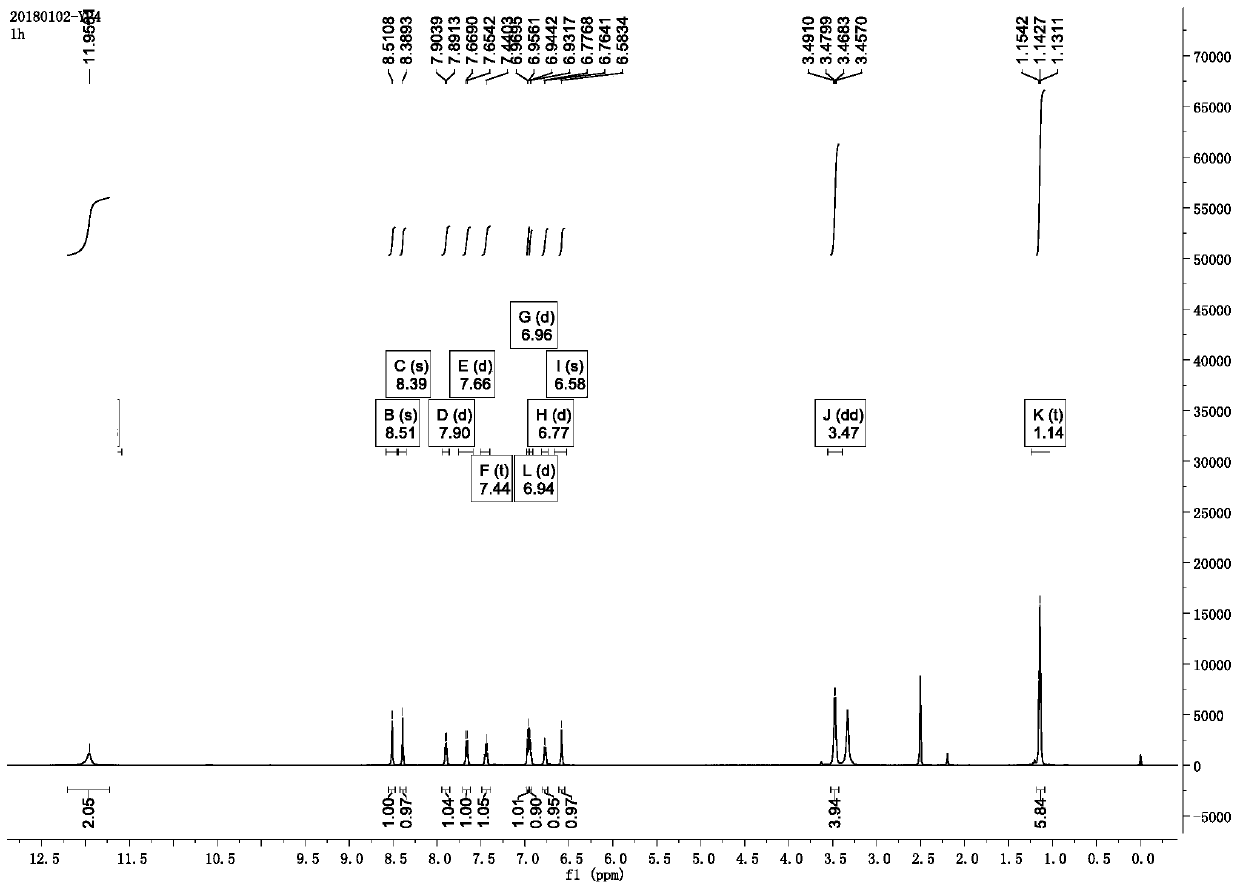
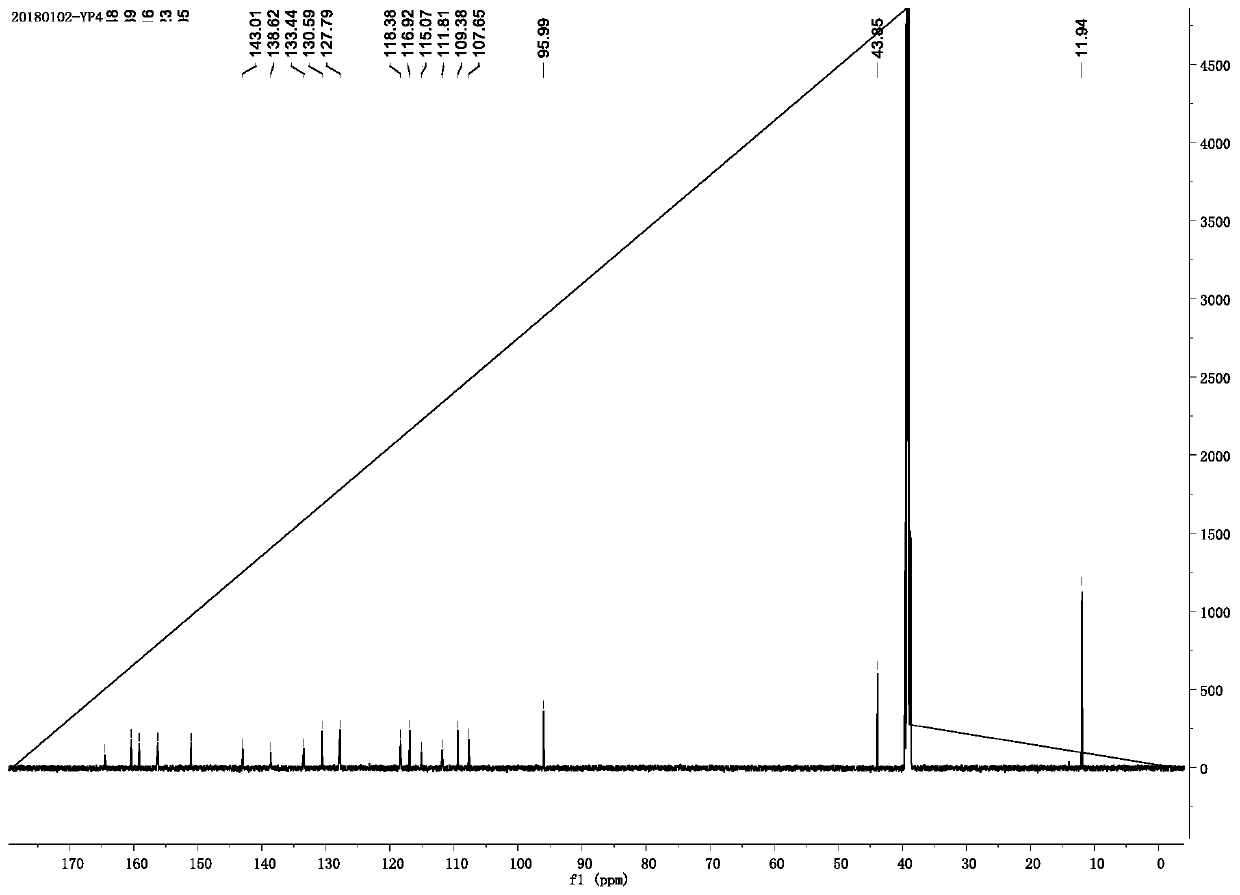
[0038] It can be determined that the product is the target small molecule fluorescent probe by mass spectrometry, NMR and spectroscopic methods, and it...
Embodiment 2
[0045] Example 2 Ultraviolet and fluorescence spectra of small molecule fluorescent probes in response to copper ions
[0046] Prepare 1.0mL small molecule fluorescent probe (1.0×10 -5 mol / L) of DMSO / H 2 O (v / v=1:1) solution. 0.5 times the normal concentration of copper ion solution was added dropwise to the probe solution, such as Figure 5 As shown in (a), after copper ions are added to the CS probe solution, the absorption bands at 316nm and 460nm gradually decrease, and there is a new absorption peak at 400nm-500nm, and the maximum absorption peak appears at 470nm. The intensity gradually increases with the ion concentration, and the final stoichiometric ratio of the two is 2:1.
[0047] In the fluorescence titration experiment, prepare 3.0mL small molecule probe (5.0×10 -6 mol / L) of DMSO / H 2 O (v / v=1:1) solution. 0.5 times the equivalent concentration of copper ion solution was added dropwise to the probe solution. For the CS probe, 460nm was used as the excitation ...
Embodiment 3
[0048] Example 3 verifies the selectivity and competitiveness of small molecule fluorescent probes for copper ions.
[0049] Prepare 5.0mL molecular probe (5.0×10 -6 mol / L) of DMSO / H 2 O (v / v=1:1) solution. Various cationic solutions [Fe(III), Al(III), Cr(III), Zn(II), Ni(II), Co(II), Mg(II) were prepared by dissolving the corresponding salts in deionized water , Ca(II), Cd(II), Mn(II), Ag(I), K(I) and Na(I)] (1.0×10 -3 mol / L). Subsequently, an equivalent equivalent of the metal ion solution was added to the probe solution. Detection is carried out by fluorescence spectroscopy, the experimental results are shown in Figure 6 (a). Take the fluorescence maximum absorption wavelength for comparison, such as Figure 6 As shown in (b), ions include Fe(III), Al(III), Cr(III), Zn(II), Ni(II), Co(II), Mg(II), Ca(II), Cd( II), Mn(II), Ag(I), K(I) and Na(I). Except for Cu(II), none of these metal ions produced significant changes in the fluorescence of the probes. After adding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com