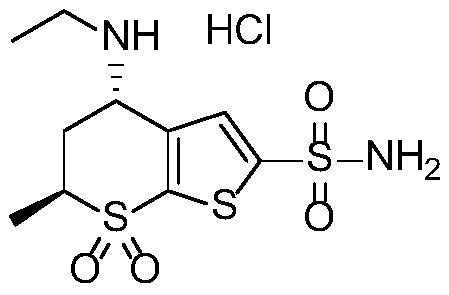
Key intermediate of dorzolamide hydrochloride and synthesis method thereof
A technique for dorzolamide hydrochloride and a synthetic method, which is applied in the chemical industry, can solve the problems of reduced production efficiency, poor chiral selectivity, increased production costs, etc., and achieves the effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
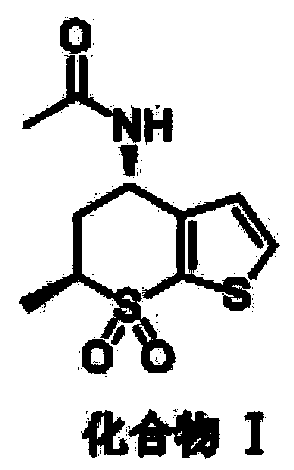
[0030] A kind of synthetic method of dorzolamide hydrochloride key intermediate, the structural formula of described dorzolamide hydrochloride key intermediate is:
[0031]
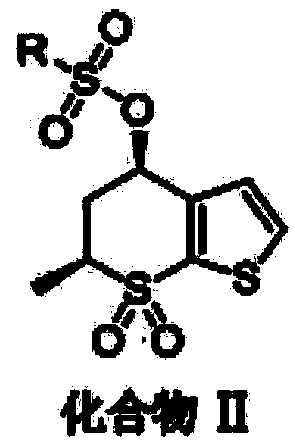
[0032] Its synthesis method is as follows: preparing compound II;
[0033] Dichloromethane 500mL and raw material (4R,6S)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-ol (cas: 147128-77-6) 50g was added to the reaction flask, and under the protection of nitrogen, the temperature was lowered to -10°C in an ice-salt bath, then 37g of methanesulfonyl chloride was added, and finally 35g of triethylamine was added dropwise to it, and the temperature was controlled React below 0°C. After the reaction is completed, add 250 mL of water to quench the reaction, stir, filter, and dry the filter cake to obtain 65 g of compound II with a yield of 96%.
[0034] The reaction chemical equation is:
[0035]
[0036] Add 65g of compound II, 65g of acetamide, 3.5g of tetrabutylammonium bromide and 65g ...
Embodiment 2
[0038] A kind of synthetic method of dorzolamide hydrochloride key intermediate, the structural formula of described dorzolamide hydrochloride key intermediate is:
[0039]
[0040] Its synthesis method is as follows: preparing compound II;
[0041] Dichloromethane 500mL and raw material (4R,6S)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-ol (cas: 147128-77-6) 50g was added to the reaction flask, and under the protection of nitrogen, the temperature was lowered to -10°C in an ice-salt bath, then 37g of methanesulfonyl chloride was added, and finally 35g of triethylamine was added dropwise to it, and the temperature was controlled React below 0°C. After the reaction is completed, add 250 mL of water to quench the reaction, stir, filter, and dry the filter cake to obtain 65 g of compound II with a yield of 96%.
[0042] The reaction chemical equation is:
[0043]
[0044] Add 65g of compound II, 80g of acetamide, 4.5g of dodecyltrimethylammonium chloride, ...
Embodiment 3
[0046] A kind of synthetic method of dorzolamide hydrochloride key intermediate, the structural formula of described dorzolamide hydrochloride key intermediate is:
[0047]
[0048] Its synthesis method is as follows: preparing compound II;
[0049] Dichloromethane 500mL and raw material (4R,6S)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-ol (cas: 147128-77-6) 50g was added to the reaction flask, and under the protection of nitrogen, the temperature was lowered to -10°C in an ice-salt bath, then 37g of methanesulfonyl chloride was added, and finally 35g of triethylamine was added dropwise to it, and the temperature was controlled React below 0°C. After the reaction is completed, add 250 mL of water to quench the reaction, stir, filter, and dry the filter cake to obtain 65 g of compound II with a yield of 96%.
[0050] The reaction chemical equation is:
[0051]
[0052]Add 65g of compound II, 100g of acetamide, 5g of 18-crown 6 and 78g of 1,8-diazabicycl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com