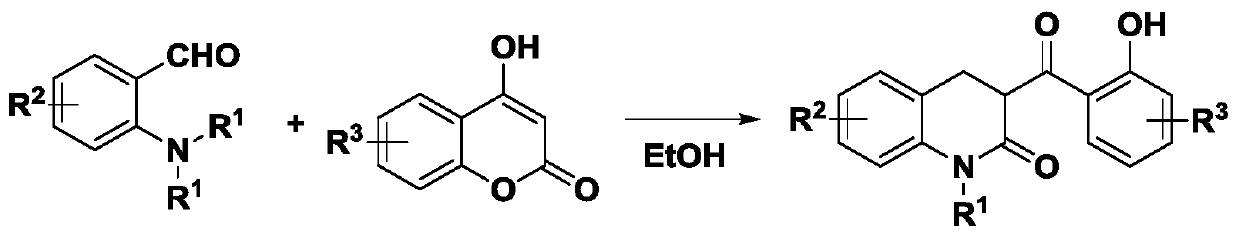
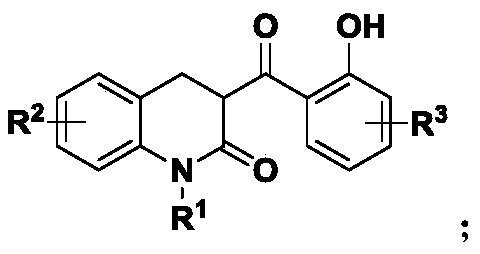
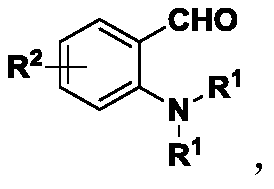
3,4-dihydro-3-(2-hydroxybenzoyl)-2(1H)-quinolinone active skeleton, and synthesis method and application thereof
A technology of hydroxybenzoyl and a synthesis method is applied in the field of 3,4-dihydro-3--2-quinolinone biologically active skeleton and its synthesis, and can solve the problems of restricting the application, restricting the industrialization of pharmaceutical production enterprises, and the like, Achieve the effect of good substrate universality, good practical significance and application value, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. This embodiment provides a method for synthesizing the bioactive skeleton of 3,4-dihydro-3-(2-hydroxybenzoyl)-2(1H)-quinolinone, which comprises the following steps:
[0044] Take 0.1 mmol of N-diethyl-substituted o-aminobenzaldehyde compounds in a reaction flask, add 1 mL of solvent in sequence, add 0.12 mmol of 4-hydroxycoumarin, and finally add the catalyst. Control the reaction temperature of the system, keep stirring, trace the reaction by spotting samples on the thin-layer chromatographic plate until the reaction of the raw materials is complete. After the reaction is completed, a silica gel column is used for separation and purification, and the purified product is rotary evaporated to obtain the target product. The reaction formula is as follows:
[0045]
[0046]2. According to the above method, set up 7 parallel test groups, using different catalysts and solvents respectively. The catalysts are p-toluenesulfonic acid monohydrate TsOH·H 2 O, triethylam...
Embodiment 2
[0057] Raw materials: N-Dimethyl-substituted anthranilaldehyde, 4-hydroxycoumarin
[0058] Product: Chemical formula: C 17 h 15 NO 3
[0059] Molecular weight: 281.1052
[0060] Structural formula:
[0061] Yield: 80%
[0062] 1 H NMRδ11.96(s,1H),7.80(d,J=7.6Hz,1H),7.49(t,J=7.3Hz,1H),7.32(t,J=7.7Hz,1H),7.19(d, J=7.3Hz, 1H), 7.11–7.03(m, 2H), 7.00(d, J=8.4Hz, 1H), 6.92(t, J=7.6Hz, 1H), 4.63(dd, J=10.4, 6.0 Hz,1H),3.49(dd,J=15.6,10.5Hz,1H),3.42(s,3H),3.10(dd,J=15.7,5.9Hz,1H). 13 C NMR (126MHz, CDCl 3 )δ201.8, 167.3, 162.9, 140.0, 136.8, 130.8, 128.0, 127.9, 124.2, 123.4, 119.4, 119.1, 118.7, 114.9, 48.3, 30.0, 28.7. HRMS (ESI): calcd for C 17 h 15 NO 3 Na[M+Na] + :304.0950,found:304.0948.
Embodiment 3
[0064] Raw materials: N,N-diethyl-6-amino-2-chlorobenzaldehyde, 4-hydroxycoumarin
[0065] Product: Chemical formula: C 18 h 16 ClNO 3
[0066] Molecular weight: 329.0819
[0067] Structural formula:
[0068] Yield: 70%
[0069] 1 H NMR (500MHz, CDCl 3 )δ11.92(s,1H),7.81(dd,J=8.1,1.1Hz,1H),7.53–7.46(m,1H),7.23(t,J=8.2Hz,1H),7.13(d,J =7.8Hz,1H),7.00(d,J=8.4Hz,2H),6.96–6.90(m,1H),4.60(dd,J=10.0,6.2Hz,1H),4.02(tq,J=14.2, 7.2Hz, 2H), 3.50(dd, J=16.4, 10.0Hz, 1H), 3.33(dd, J=16.4, 6.2Hz, 1H), 1.28(t, J=7.1Hz, 3H). 13 C NMR (125MHz, CDCl 3 )δ201.3, 166.0, 162.9, 140.1, 136.9, 133.6, 130.8, 128.3, 124.3, 123.0, 119.3, 119.2, 118.7, 113.4, 47.6, 38.4, 25.6, 12.6. HRMS (ESI): calcd for C 18 h 16 ClNO 3 Na[M+Na] + :352.0711,found:352.0708.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com