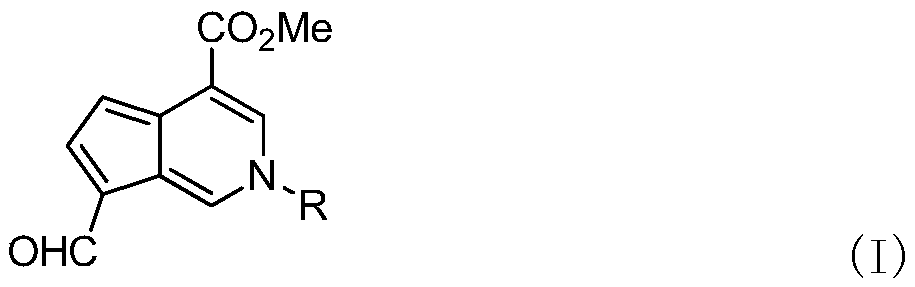Aromatic cyclopentenopyridine as well as synthesis method and application thereof
A technology of cyclopentenopyridine and synthesis method, which is applied in the field of pesticides, can solve the problems of limited synthesis and application, and achieve the effect of inhibiting tobacco mosaic virus and excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
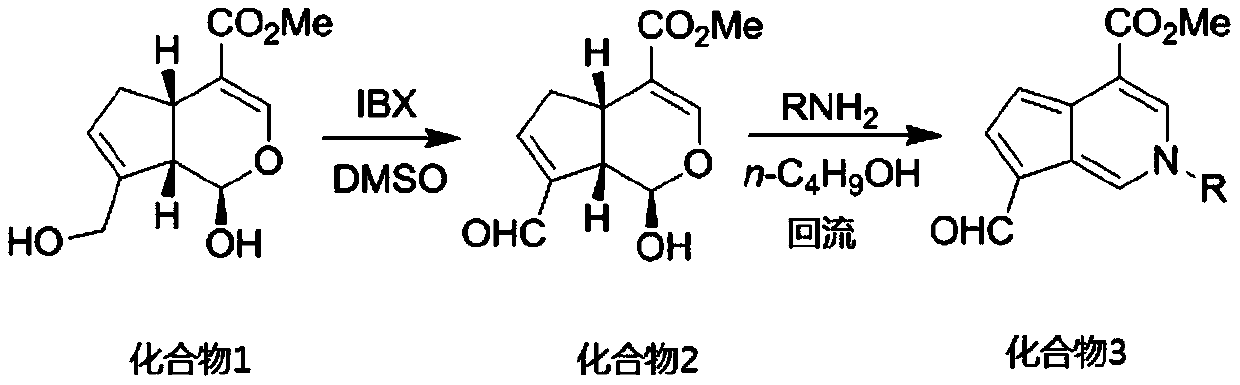
[0029] 8.00 g (35.4 mmol) of genipin, 10.89 g (38.9 mmol) of 2-iodobenzoic acid (IBX) and 200 ml of dimethyl sulfoxide were added to a 500 mL round bottom flask. Under argon protection, after electromagnetic stirring at room temperature for 3 h, 300 mL of water was added. Suction filtration, the filtrate was extracted four times with ethyl acetate, the organic phases were combined, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, and precipitated to obtain the crude product of 2, and the pure product of compound 2 was obtained by column chromatography.
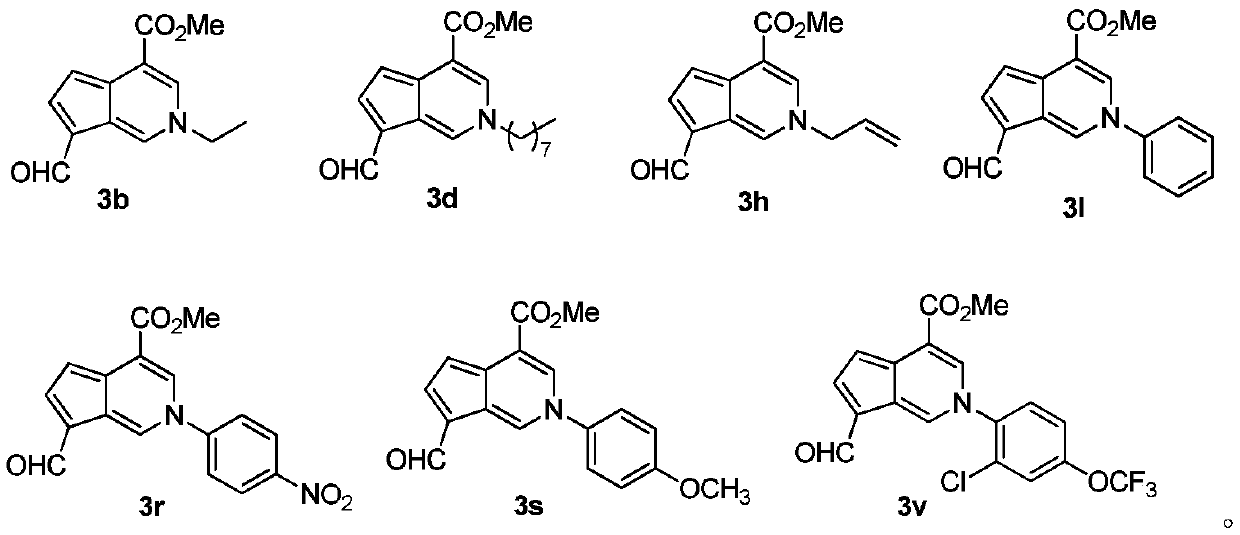
[0030] Compound 2 (1.00 g, 4.46 mmol), primary amine (4.46 mmol) and 60 mL of n-butanol were added to a 100 mL round bottom flask. After heating to reflux for 5 h, n-butanol was removed with a rotary evaporator. The target compound 3 was obtained by neutral alumina column chromatography, that is, the mangolin derivative—aromatic cyclopentenopyridine.
[0031]
[003...
Embodiment 2
[0055] Biological activity Take the activity of anti-tobacco mosaic virus (TMV) as an example,
[0056] 1. Virus purification and concentration determination:
[0057] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0058] 2. Preparation of compound (mangolin derivatives - aromatic cyclopentenopyridine) solution:
[0059] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted to the required concentration with an aqueous solution containing 1‰ Tween 80; ribavirin preparations were directly diluted with water.
[0060] 3. In vivo protection:
[0061] Select Shanxi tobacco with uniform growth at the 3-5...
Embodiment 3
[0077] Insecticidal activity test:
[0078] 1. Activity test of diamondback moth larvae
[0079] The leaf dipping method proposed by the International Resistance Action Committee (IRAC) was used. Weigh 2 mg of drug sample on an analytical balance into a 10 mL small beaker, add 50 μL of dimethylformamide (analytical grade) to dissolve, add 10 mL of water to make a 200 mg / kg drug solution. Immerse the cabbage leaves with straight ophthalmic tweezers for 2-3 seconds, and shake off the remaining liquid. 1 tablet each time, 3 tablets in total for each sample. Place the samples on the processing paper in the order they are marked. After the liquid medicine dries, put it into a marked 10cm long straight tube, insert the 2nd instar diamondback moth larvae, and cover the tube mouth with gauze. Put the test treatment in the standard treatment room, and check the results after 96 hours. Each compound was repeated 3 times. For the control, only emulsifier and solvent were added to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com