Inhibitors of bruton's tyrosine kinase
An amino, solvate technology, applied in the field of medicines for the treatment of diseases and disorders, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414] Example 1. General Synthesis of Compounds of Formula I.
[0415]
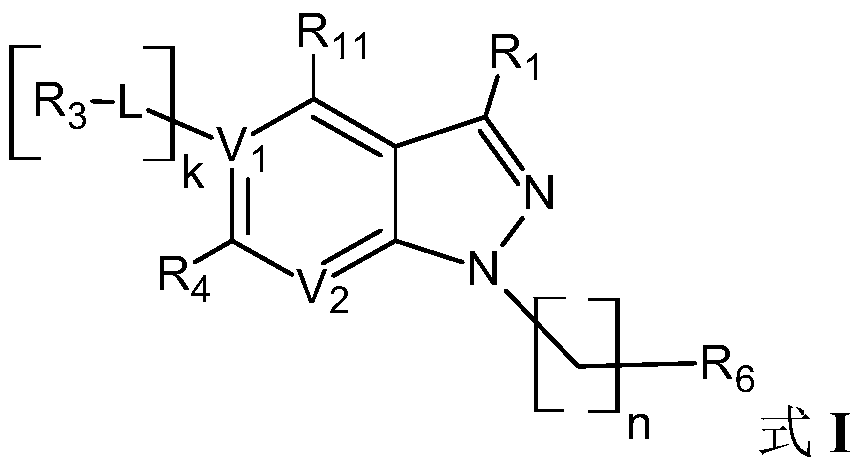
[0416] where V 1 , V 2 , L, R 1 , R 3 , R 4 , R 11 , n, k have the above meanings.
[0417] General Synthetic Procedures for Compounds of Formula III.
[0418]
[0419] where V 1 , V 2 , L, R 1 , R 3 , R 4 , R 11 , n, k have the above meanings.
[0420] Step 1: Synthesis of compound B(E). In a three-necked flask equipped with a stirrer and thermometer, mix under nitrogen in the indicated order: 20 mL of 1,4-dioxane; (0.002 mol) the necessary compound X1, X2 or X3; 0.759 g (0.003 mol) ) of bis(pinacolate) diboron; 0.190 g (0.0004 mol) of XPhos; 0.588 g (0.006 mol) of dried potassium acetate; 0.067 g (0.0002 mol) of palladium(II) acetate. While stirring, an inert gas (argon or nitrogen) was passed through the mixture for 15 minutes. The resulting reaction was stirred at 80-90° C. for 3-5 hours under inert gas; TLC method was used to ensure completion of the reaction. When the reactio...
Embodiment 2
[0427] Example 2. The general synthesis method of compounds X1, X2, X3.
[0428]
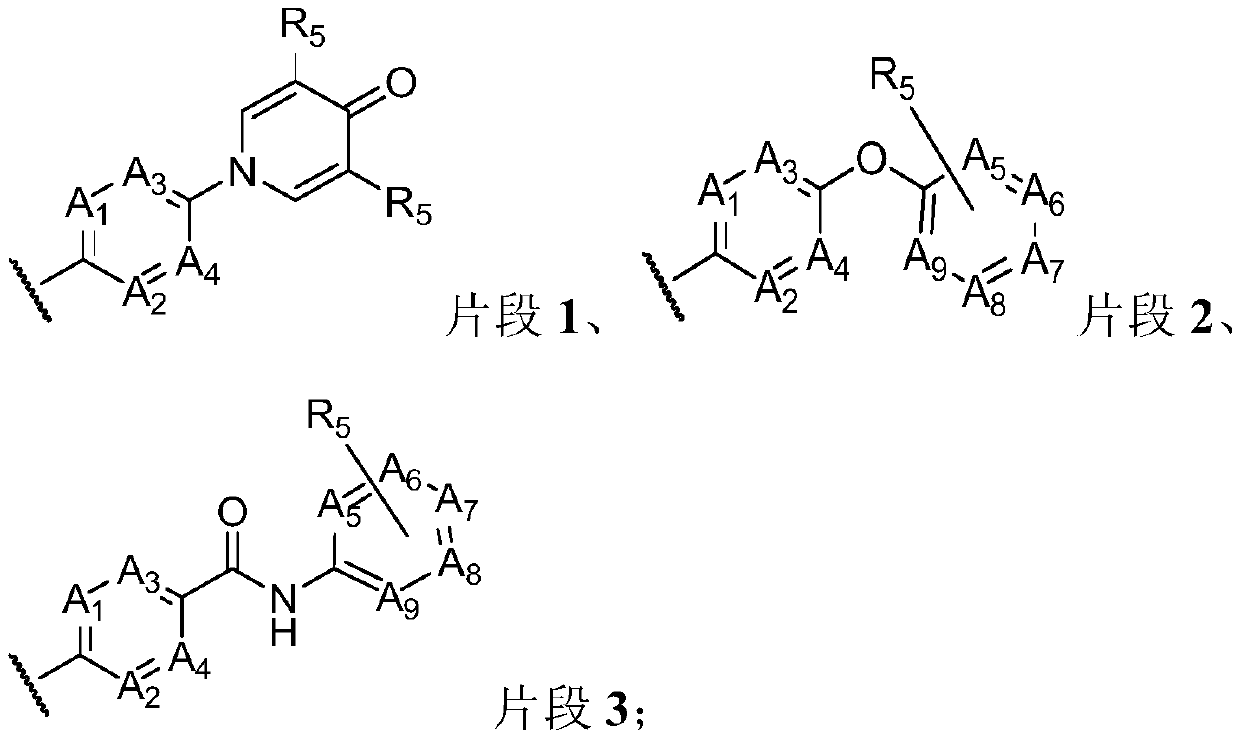
[0429] where A 1 、A 2 、A 3 、A 4 、A 5 、A 6 、A 7 、A8 、A 9 , R 5 has the above meaning.
[0430] Compound X1. In a round bottom flask equipped with a stirrer, thermometer and reflux condenser, mix in the indicated order: 200 mL of DMF, 0.1 mol of the corresponding dihalogenated benzene X1-2, 0.1 mol of the corresponding hydroxypyridine X1-1 and 0.2 mol of cesium carbonate or potassium carbonate. The mixture was stirred at 100° C. for 2-6 hours under inert gas; TLC method was used to ensure completion of the reaction. Then, most of the solvent was distilled off using a rotary evaporator; 200 mL of ethyl acetate was added, and the resulting suspension was filtered through celite. The filtrate was evaporated. The resulting product was purified by column chromatography, eluent: ethyl acetate:methanol (9:1). The product obtained is the compound of formula X1 in 60% to 80% yield.
[0431...
Embodiment 3
[0433] Example 3. Method for Synthesizing Intermediates.
[0434] 1)
[0435]
[0436] BCD-BTK-4-11. In a round bottom flask equipped with a stirrer, thermometer and reflux condenser, dissolve 20.6g (0.158mol) of 2-amino-4-chloropyridine in tert-butanol, add 38.5 g (0.175 mol) of BOC anhydride. The mixture was stirred at 40°C for 5 hours. Excess solvent was distilled off by rotary evaporator at 40°C; the residue was treated with hexane. The resulting suspension was cooled to 0°C and the precipitate was filtered off. Yield: 28 g (77%).
[0437] BCD-BTK-4-10. In a round bottom flask equipped with stirrer, thermometer, and reflux condenser, mix in the order indicated: 135 mL of dry tetrahydrofuran (THF), 20 g (0.169 mol) of N,N,N' , N'-tetramethylethylenediamine and 15.7 g (0.068 mol) of BCD-BTK-4-11. The reaction mixture was cooled to -78°C; 68 mL of 2.5M n-butyllithium in hexanes was added dropwise, maintaining the temperature. Then, the reaction mass was allowed to s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com