Application of gastrodin in preparing drugs for treating cerebral hemorrhage
A gastrodin and drug technology, applied in the field of medicine, can solve problems such as hemorrhagic transformation that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
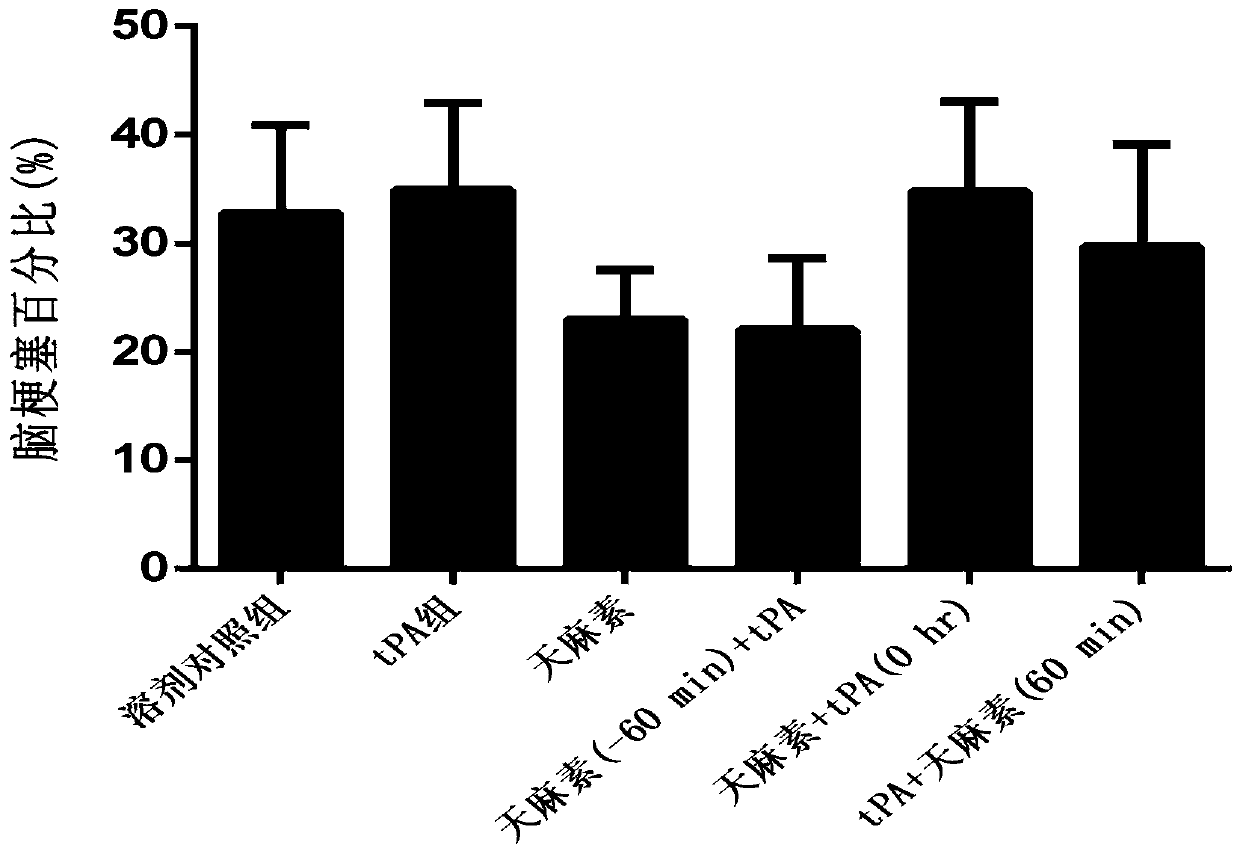
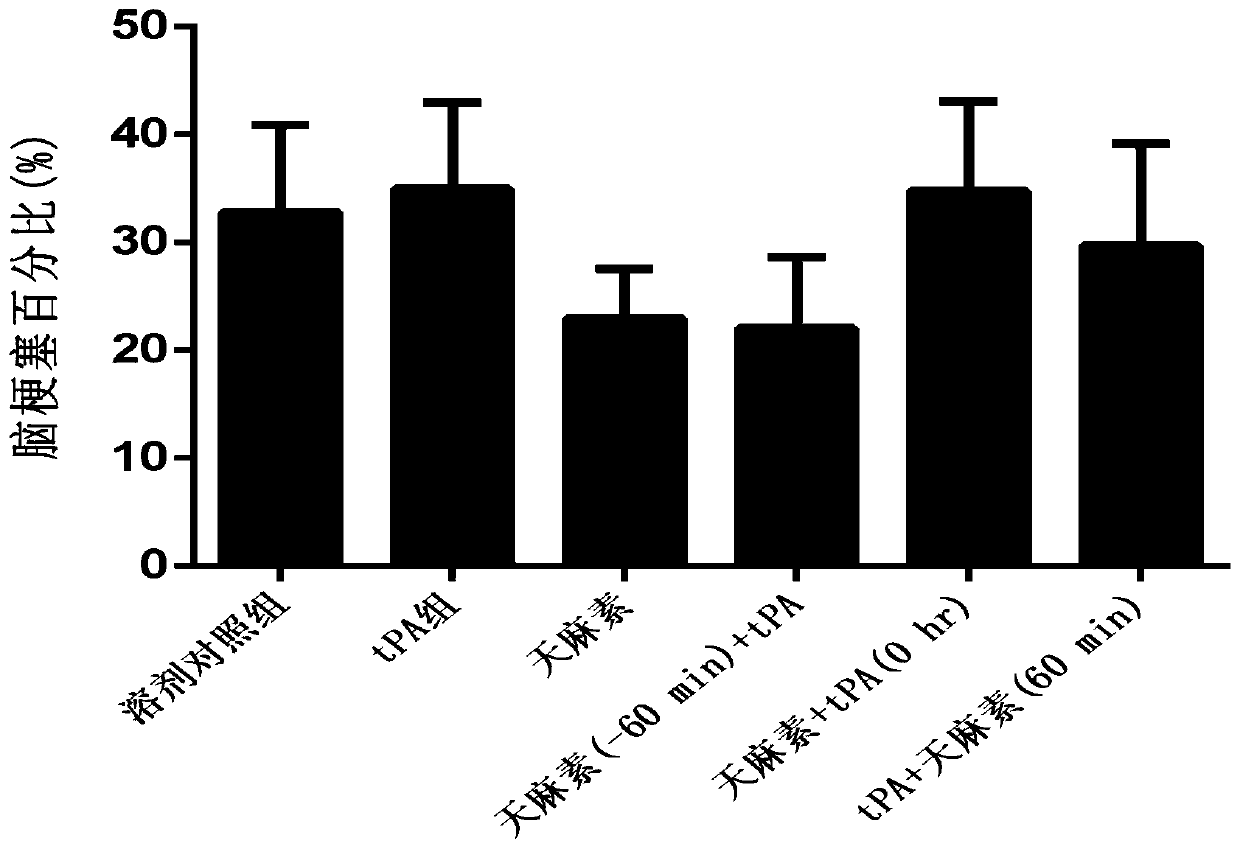
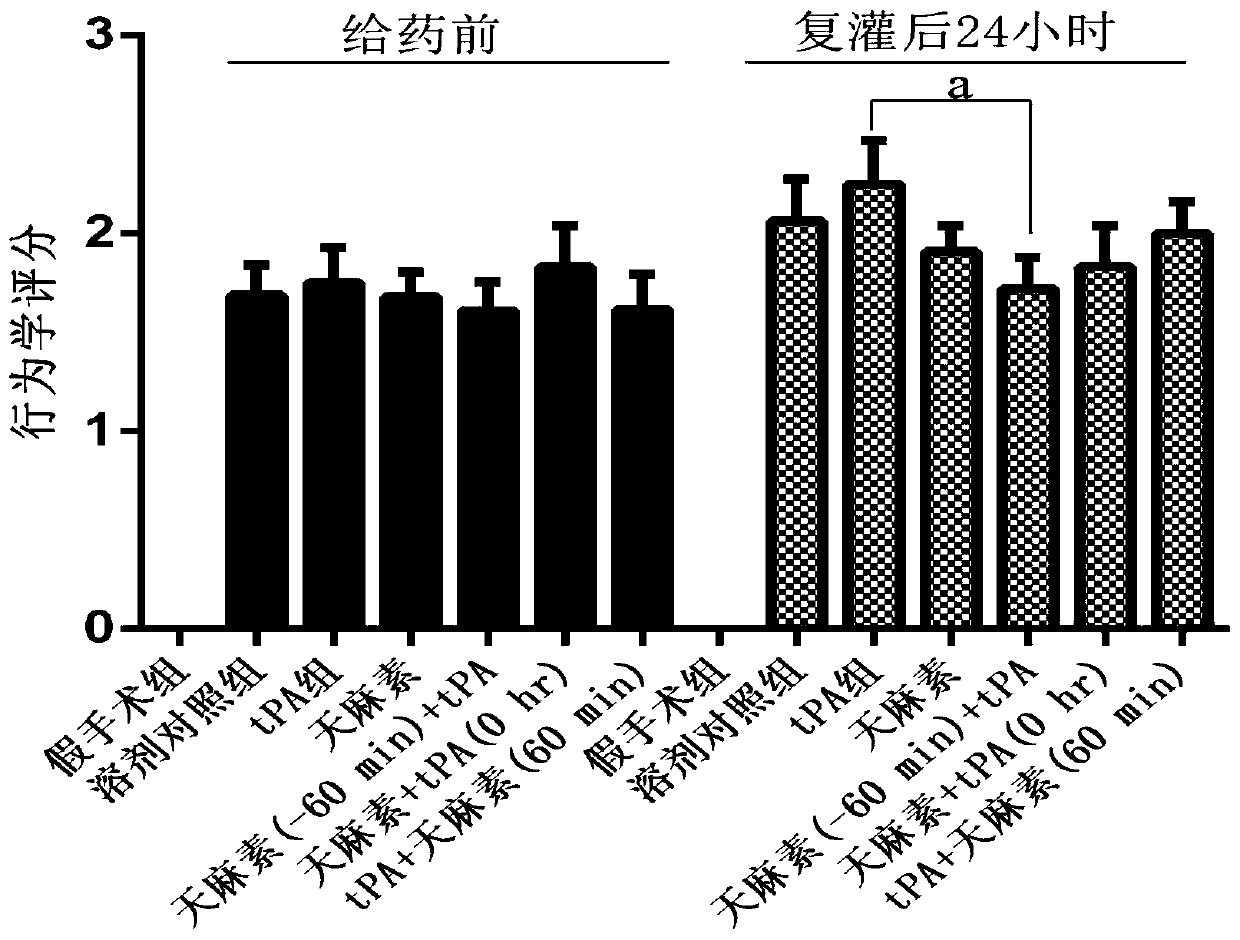
[0049] Example 1: Study on the protective effect of gastrodin on hemorrhagic transformation in rats with cerebral ischemia-reperfusion induced by rt-PA
[0050] 1. Reagents and animals
[0051] 1.1 Test product: gastrodin injection, Kunming Pharmaceutical Group Co., Ltd., traits: colorless transparent liquid; specification: 2mL: 0.2g / bottle; batch number 17JX06-222; storage conditions: room temperature, dark; expiration date: 2020.09;
[0052] 1.2 Reference substance: name or code: alteplase (rt-PA); Boehringer Ingelheim (Germany); traits: off-white loose lumps; specification: 20mg / bottle (including diluent); batch number: 703948; storage conditions: 2 ~ 8 ℃, airtight, away from light; validity period: 2020.02;
[0053] 1.3 Other reagents: Hemoglobin Quantitative Kit (BioAssay Systems, USA, Cat: DIHB-250); MMP9 ELISA Detection Kit (R&D, USA, Cat: RMP900); Rat Cytokine / Chemokine (TNF-α, IL-6, IL -12(p70), IL-1beta) liquid phase chip, manufacturer: Millipore, USA, Cat: RECYTNA...
Embodiment 2
[0127] Example 2: Study on the protective effect and mechanism of gastrodin on HBMEC induced by OGD
[0128] 1. Experimental materials
[0129] 1.1 Test product: gastrodin injection, Kunming Pharmaceutical Group Co., Ltd., traits: colorless transparent liquid; specification: 2mL: 0.2g / bottle; batch number 17JX06-222; storage conditions: room temperature, dark; expiration date: 2020.09;
[0130] 1.2 Reference substance: name or code: nimodipine injection, batch number: 180320, specification: 20mL: 4mg, Yabao Pharmaceutical.
[0131] 1.3 Experimental cells: human brain microvascular endothelial cell line (HBMEC), purchased from Guangzhou Geneo Biotechnology Co., Ltd.
[0132] 1.4 Other reagents: endothelial cell medium (ECM), ScienCell; high-sugar DMEM basal medium, sugar-free DMEM basal medium, DPBS Duchenne's phosphate buffer, PBS phosphate buffer, fetal bovine serum (FBS), 0.25% Trypsin- EDTA (1X) was purchased from Gibco; penicillin-streptomycin (double antibody), BI; dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com